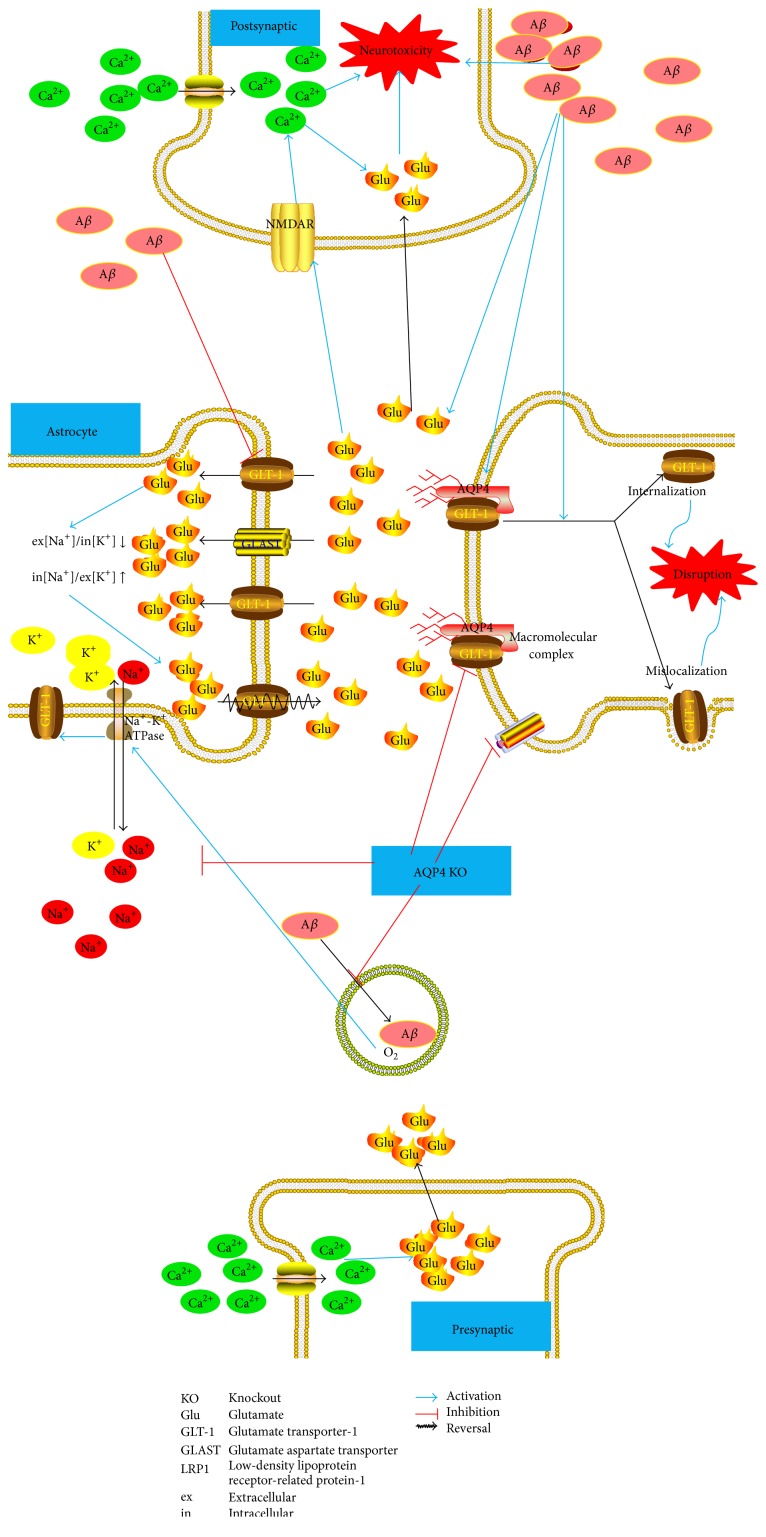
Figure 1.
Neuroprotective effect of the association of AQP4/GLT-1 against glutamate-induced neuronal injury by Aβ. (1) Aβ targets. Aβ may increase the release of physiological synaptic glutamate. This in turn results in neuronal NMDAR activation, which leads to synaptic damage and neurotoxicity. Glutamate uptake by astrocytes is also reduced after Aβ infusion, which results in high extracellular glutamate concentrations, and, consequently, excitotoxicity. Glutamate dyshomeostasis could play a role in the pathogenesis of AD, and in this process, GLT-1 has demonstrated to undergo oxidative damage by exposure to Aβ. (2) Glutamatergic system. GLT-1 is the major glutamate transporter that is responsible for various essential neuroprotective functions that include the prevention of glutamate-mediated injury of neurons and synapses; it accomplishes this through the transport of glutamate in the inward direction under normal conditions. In excitotoxic conditions, when the extracellular [Na+]/intracellular [K+] ratio is decreased and/or when the intracellular [Na+]/extracellular [K+] ratio is increased, glutamate is transported in the outward direction, such that GLT-1 reversal might further intensify glutamate-induced synaptic damage. (3) Consequences of AQP4 knockout. It is known that AQP4 deficiency may downregulate both GLT-1 expression and glutamate uptake, but it may also downregulate LRP1, which is involved in Aβ clearance. Moreover, AQP4 deficiency could cause the inhibition of oxygen delivery from brain microvessels to the Na+/K+-ATPase. This might represent another important factor in the regulation of Na+/K+-ATPase function and ultimately the neuroprotection of GLT-1 against AD toxicity. (4) Neuroprotective effects of the association of AQP4 and GLT-1. As a functional complex, AQP4 and GLT-1 in astrocyte cell membranes could exert neuroprotective effects, and the mislocalization and internalization of GLT-1 in astrocyte membranes, as promoted by Aβ, might be responsible for the disruption of this macromolecular complex. This leads to a marked reduction in the rate at which astrocytes clear glutamate from the extracellular space, and, most importantly, to the development of AD.