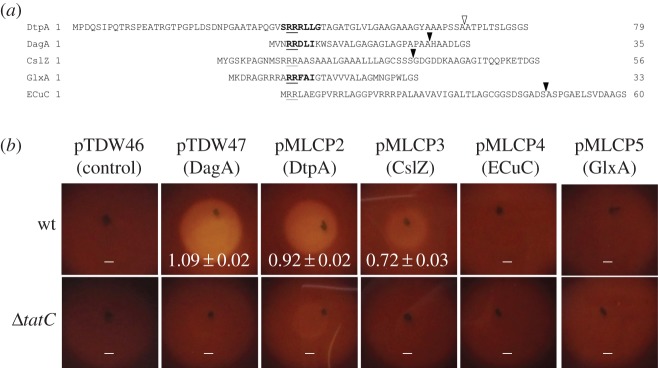
Figure 6.
Tat-dependent protein secretion of DtpA and CslZ in S. lividans. (a) N-terminal signal sequences of the S. lividans DtpA, DagA, CslZ, GlxA and ECuC proteins. Boldface text highlights the twin-arginine motifs predicted by TatP, with the conserved arginines being underlined. The solid triangles at the C-termini indicate the predicted strong peptidase cleavage sites, while the open triangle indicates the predicted cleavage site in DtpA, which has a low cleavage site score. The amino acids Gly–Ser at the end of the signal sequences result from the introduced BamHI restriction site used for their cloning. (b) Visualization of extracellular agarase activity after lugol staining of S. lividans strains grown on MM-C medium with agar as the sole carbon source. The used strains expressed the DagA protein without its signal sequence (pTDW46), or with signal sequences of DagA (pTDW47), DtpA (pMLCP2), CslZ (pMLCP3), ECuC (pMLCP4) of GlxA (pMLCP5). Halos are indicative for DagA secretion. No halos were observed when the constructs were introduced in the tatC mutant. Numbers indicate the mean diameter of clearing zones in cm with the corresponding standard error of the mean.