Figure 3.
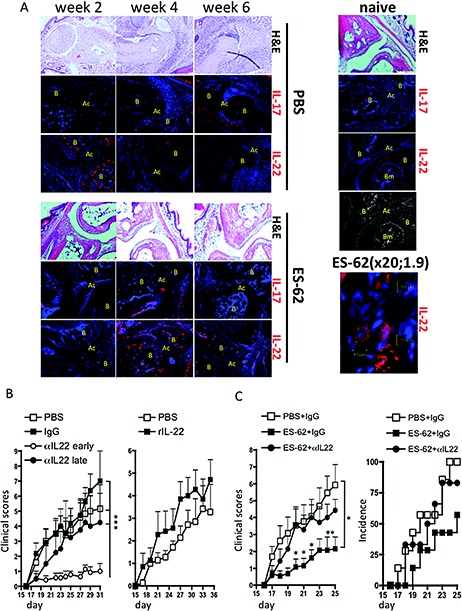
ES‐62 protection against CIA requires IL‐22. A, Joint sections from representative PBS‐treated mice with CIA, ES‐62–treated mice with CIA, and naive mice at weeks 2, 4, and 6 (articular scores 0, 4, and 8, respectively, at weeks 2, 4, and 6 in the PBS‐treated mice and 0, 2, and 2, respectively, in the ES‐62–treated mice). Sections were stained with hematoxylin and eosin (H&E) or for IL‐17 or IL‐22 (red) or nuclei (DAPI; blue). Isotype control sections were negative for IL‐17 and IL‐22. Joint structure is shown in the grayscale image. B = bone; AC = articular cavity; BM = bone marrow. Original magnification × 20 (1.9 zoom). B, Mean ± SEM articular scores in mice with CIA treated with PBS (n = 12), murine IgG (100 μg/dose; n = 11), or anti–IL‐22 (100 μg/dose) twice weekly from day 7 (early αIL‐22; n = 14) or from day 19 (late αIL‐22; n = 4), or treated intraperitoneally (IP) with PBS (n = 7) or recombinant IL‐22 (rIL‐22) (1 μg/dose; n = 7) twice weekly from day 7. ∗∗∗ = P < 0.001. C, Mean ± SEM articular scores in mice with CIA treated with PBS plus IgG (n = 13), ES‐62 plus IgG (n = 13), or ES‐62 plus anti–IL‐22 (n = 12) and with antibodies administered IP twice weekly from day 19 onward (100 μg/dose) and ES‐62 administered on days −2, 0, and 21 (2 μg/dose), and percent incidence of disease (score >1) by treatment group. ∗ = P < 0.05 (P values shown for specific days are versus treatment with ES‐62 plus anti–IL‐22). See Figure 1 for other definitions.
