Figure 3.
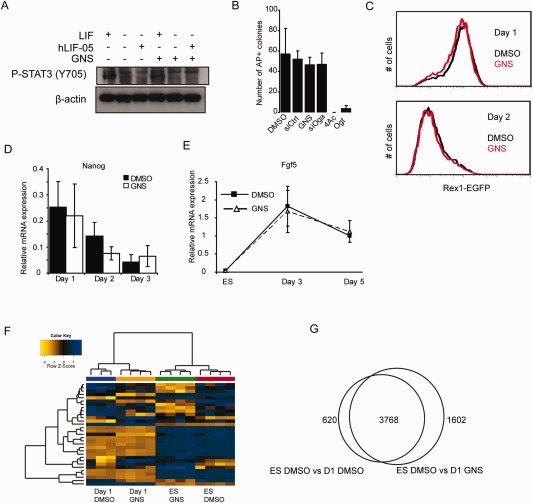
Increased O‐GlcNAcylation does not affect naïve‐primed embryonic stem cell (ESC) transition. (A): 46C ESCs were cultured in the absence of LIF overnight and then stimulated with either LIF or the LIF receptor antagonist hLIF‐05 in the presence of either DMSO or GNS, then blotted for phospho‐Stat3 (Y705). GNS treatment does not interfere with the ability of LIF to stimulate Stat3 phosphorylation and does not cause Stat3 phosphorylation in the absence of LIF. (B): GNS or Oga knockdown does not affect the ability of ESCs to form undifferentiated colonies, while inhibition or knockdown of Ogt results in cell death. 4Ac: 4‐Acetyl‐5S‐GlcNAc (Ogt inhibitor). (C): OCRG9 ESCs (Rex1EGFP reporter line) were differentiated in N2B27 for 2 days in vehicle or GNS and analyzed by flow cytometry for EGFP fluorescence. GlcNAcstatin treatment does not affect the loss of naïve marker Rex1. (D): Quantitative RT‐PCR analysis during monolayer differentiation shows that expression of the naïve marker Nanog is lost at equivalent rates in the presence of vehicle (DMSO; black bars) or GNS (white bars). (E): Upregulation of the primed pluripotency marker Fgf5 is unaffected by GNS treatment (D and E, n = 3, mean ± SEM). (F): Hierarchical clustering (using Euclidean distance with complete linkage agglomeration method) of samples based on the top 31 differentially expressed genes (ranked by p‐value) separates the samples according to the stage of differentiation and shows clustering of genes that contribute to differentiation. (G): Pairwise comparison of genes regulated during the first 24 hours of differentiation in vehicle (DMSO) or GNS (fold change >1.2, p < .05). Abbreviation: GNS, GlcNAcstatin C.
