Figure 1.
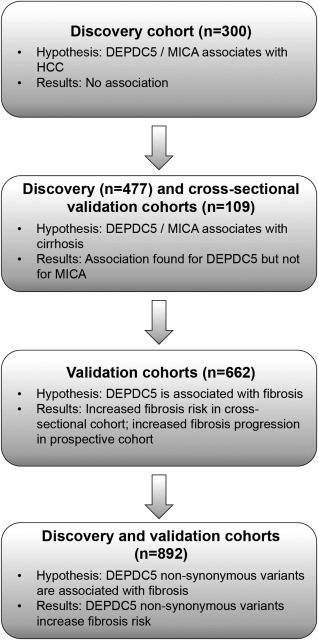
Study design and flow of the analyses. This picture illustrates the study design and flow of analyses. Both the discovery and the validation cohorts consist of therapy‐naïve subjects with chronic HCV infection. The discovery cohort consisted of a cross‐sectional group of 477 subjects enrolled in Milan (Italy). In the analysis on HCC, only patients with cirrhosis were included. To test the association with fibrosis, we first analyzed the extremes of fibrosis distribution (stage F0‐F1 versus F4) to maximize the power by selecting two extremely different groups. Next, we examined the association with the full spectrum of fibrosis in the cross‐sectional validation cohort (the Leipzig and Bern cohorts pooled together [n = 415]) and with fibrosis progression in the prospective cohort (Milan prospective cohort [n = 247]). Only the significant associations were carried on in the validation cohorts. The analysis of nonsynonymous variants was performed by pooling together the discovery and the cross‐sectional validation cohorts.
