Figure 2.
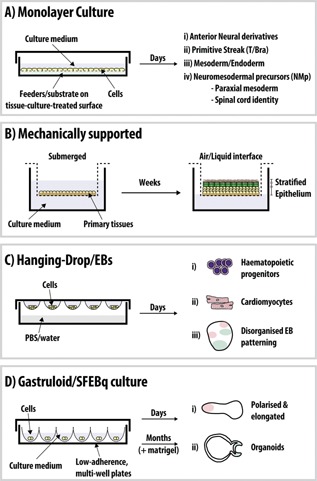
Comparison of culture methods. Schematics of the typical culture methods utilised for the differentiation of SCs. A: Cells grown as a monolayer on a bed of feeders or surfaces coated with substances such as gelatin or fibronectin. In the case of ESCs, specific culture conditions can direct their differentiation towards anterior neural (i), a primitive streak (PS) population (e.g. T/Bra‐expressing cells 66; ii), derivatives of the germ layers (iii) and a neuromesodermal progenitor (NMp) population for axial tissues such as the spinal cord and paraxial mesoderm 74, 98. B: Mechanically supported culture allows the further differentiation of primary tissues such as human keratinocytes. Upon contact with an air‐liquid interface and over a period of weeks, cells differentiate and self‐assemble to form a fully stratified tissue (adapted from 15). C: Embryoid bodies (EBs) can either be generated on low‐adherence tissue‐culture plastic or through hanging drop culture (pictured). In the latter case, droplets of ESCs are suspended above PBS or water and cultured for a number of days. Haematopoietic progenitors (i) 23 and cardiomyocytes 22 (ii) have been produced through EB culture. EBs typically show disorganised gene expression (iii), however polarised, elongated structures have been formed by this method using low numbers of EC cells 75. D: More modern techniques producing “Gastruloids” (i) and the serum‐free floating culture of embryoid‐body‐like aggregates with quick reaggregation (SFEBq) 27 (ii) have been successful in generation of structures that mimic a number of early developmental processes (axial elongation, polarisation; (i) as well as the generation of self‐assembling and patterned organoids such as the optic cup (ii). In the case of the latter organoids, cells are usually embedded in Matrigel and occasionally transferred to bacterial dishes once aggregation has occurred. See Table 1 for details on the culture methods and time for organoid formation.
