Fig. 1.
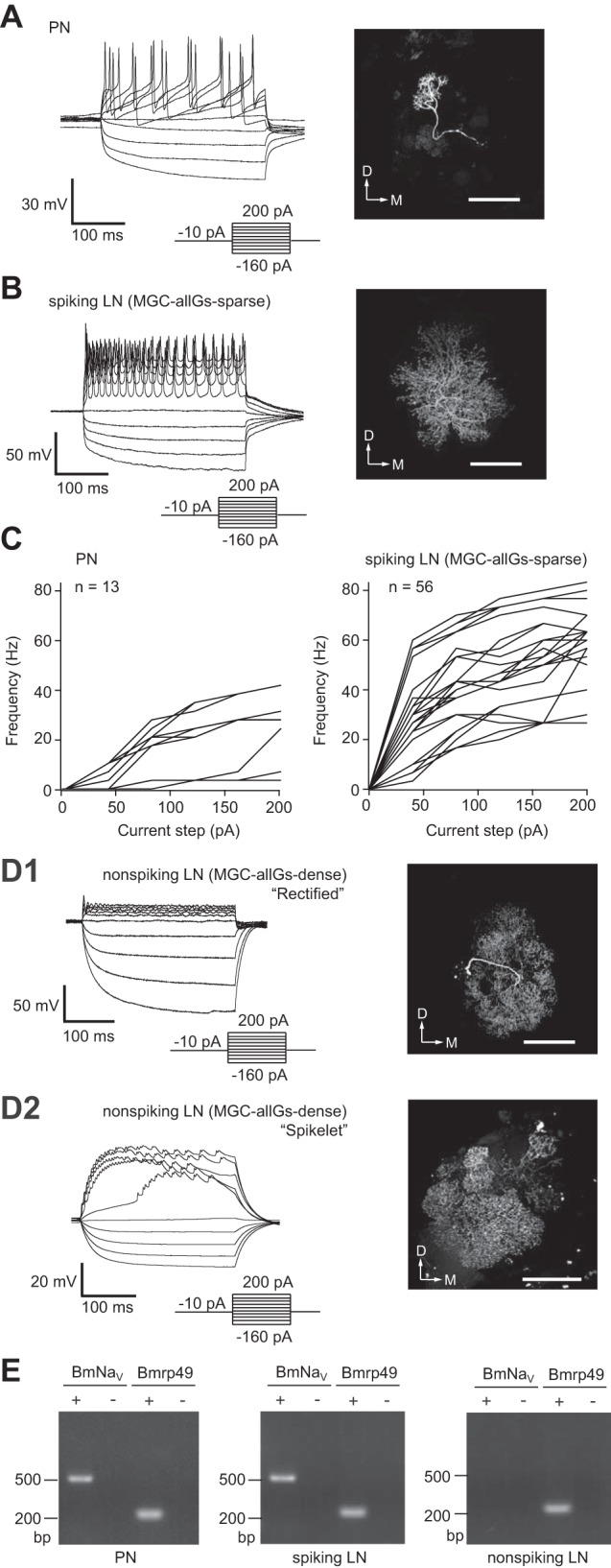
Distinct physiological properties with two morphologically distinct local interneurons (LNs) and projection neuron (PN). A: whole cell current-clamp recordings of PNs showing their typical membrane voltage responses to current steps (left) and the morphology of a recorded neuron, which is visualized by filling with biocytin (right). D, dorsal; M, medial. Scale bar, 100 μm. B: whole cell current-clamp recordings of spiking LNs showing their typical membrane voltage responses to current steps (left) with MGC-allGs-sparse morphology (global multiglomerular type of LN that arborizes in both the MGC and most ordinary glomeruli; see text), which is visualized by filling with biocytin (right). Scale bar, 100 μm. C: mean firing rate vs. injected current for PNs (left) and for spiking LNs (right). The mean firing frequency of action potentials during the current injection was calculated (n = 13 for PNs; n = 56 for LNs). D: whole cell current-clamp recordings of nonspiking LNs and their MGC-allGs-dense morphology, which is visualized by filling with biocytin. D1: “rectified” response of nonspiking LNs showing strong rectification in response to a depolarizing current injection (left) and the MGC-allGs-dense morphology of the recorded neuron (right). D2: “spikelet” response of nonspiking LNs showing a tetrodotoxin (TTX)-insensitive mini-spike waveform in response to a depolarizing current injection (left) and the MGC-allGs-dense morphology of the recorded neuron (right). Scale bars, 100 μm. E: single-cell RT-PCR analysis of the voltage-gated sodium channel gene (BmNaV). Single-cell RT-PCR was conducted with total RNA from PNs and spiking and nonspiking LNs. The minus sign indicates a negative control without reverse transcriptase. The ribosomal protein gene (Bmrp49) was used as a positive control.
