Abstract
BACKGROUND
There is interest in newborn screening and diagnosis of lysosomal storage diseases because of the development of treatment options that improve clinical outcome. Assays of lysosomal enzymes with high analytical range (ratio of assay response from the enzymatic reaction divided by the assay response due to nonenzymatic processes) are desirable because they are predicted to lead to a lower rate of false positives in population screening and to more accurate diagnoses.
METHODS
We designed new tandem mass spectrometry (MS/MS) assays that give the largest analytical ranges reported to date for the use of dried blood spots (DBS) for detection of mucopolysaccharidoses type II (MPS-II), MPS-IVA, and MPS-VI. For comparison, we carried out fluorometric assays of 6 lysosomal enzymes using 4-methylumbelliferyl (4MU)-substrate conjugates.
RESULTS
The MS/MS assays for MPS-II, -IVA, and -VI displayed analytical ranges that are 1–2 orders of magnitude higher than those for the corresponding fluorometric assays. The relatively small analytical ranges of the 4MU assays are due to the intrinsic fluorescence of the 4MU substrates, which cause high background in the assay response.
CONCLUSIONS
These highly reproducible MS/MS assays for MPS-II, -IVA, and -VI can support multiplex newborn screening of these lysosomal storage diseases. MS/MS assays of lysosomal enzymes outperform 4MU fluorometric assays in terms of analytical range. Ongoing pilot studies will allow us to gauge the impact of the increased analytical range on newborn screening performance.
Newborn screening (NBS)5 and diagnosis of lysosomal storage diseases (LSD) are under investigation because of the development of treatment options (1). Assay of the activity of the deficient enzyme in dried blood spots (DBS) on NBS cards was first carried out with fluorometric methods based on 4-methylumbelliferyl (4MU) substrates for disease such as Fabry disease (2) and more recently with tandem mass spectrometry (MS/MS) (3). Methods based on both technologies are being piloted for worldwide NBS and diagnosis (3).
Mucopolysaccharidoses are a family of LSDs for which the deficiency is in the breakdown of glycosaminoglycans (4). For mucopolysaccharidosis-II (MPS-II), MPS-IVA, and MPS-V, the deficient enzymes are, respectively, iduronide-2-sulfatase (I2S), N-acetylgalactosamine-6-sulfatase (GALNS), and N-acetylgalactosamine-4-sulfatase (ARSB). I2S can be assayed fluorometrically with the 4MU glycoside of iduronic acid-2-sulfate using human α-L-iduronidase (IDUA) to liberate 4MU after the 2-sulfate is removed (5) or by MS/MS (6). GALNS can be assayed with the 4MU glycoside of galactose-6-sulfate using bacterial β-galactosidase to release the 4MU after the sulfatase acts (7) or by MS/MS (8). ARSB is assayed using the generic sulfatase substrate 4MU-sulfate (9) or by MS/MS (10).
We report the development of MS/MS assays for I2S, GALNS, and ARSB that give a much higher assay response in the mass spectrometer than previously reported assays. These new reagents lead to a larger lysosomal enzyme assay analytical range, which we defined as the ratio of assay response with the high QC DBS, because of the relevant enzymatic reaction, divided by the response for nonenzymatic processes. Increasing the analytical range is important for NBS and diagnosis of LSDs because this is predicted to lead to a more accurate enzyme activity value at the low end. This is expected to lead to better differentiation between disease-affected patients and those with pseudodeficiencies and, in general, lead to a lower rate of false positives. For diagnosis, it may lead to better prediction of disease severity. We also compared the analytical range of 6 MS/MS assays to those measured fluorometrically with 4MU-substrates.
Methods
All methods, including the synthesis of the substrates, are described in the Data Supplement that accompanies the online version of this report at http://www.clinchem.org/content/vol61/issue11.
Results
FIA-MS/MS ASSAYS FOR GALNS AND ARSB
Our original MS/MS substrate for GALNS consisted of a Gal-6-sulfate linked to an analog of 4MU bearing a hydrophobic chain (8). Although this assay distinguished between healthy and MPS-IVA samples, the MS/MS signal for the product in assays with random newborns was 50-fold less than for our other MS/MS assays for lysosomal enzymes. This original assay is not sufficiently robust for NBS, and the only way forward is to find a higher activity substrate or to increase the MS/MS response of the product. GALNS is thought to be responsible for removal of sulfate from Gal-6-sulfate and GalNAc-6-sulfate in mucopolysaccharides (4). Thus, we explored the consequence of replacing Gal-6-sulfate in our previous substrate with GalNAc-6-sulfate. We also replaced the 4MU-based aglycone in our original GALNS substrate with an aglycone containing the 4-acetamido-phenol moiety because this aglycone change results in an improved assay MS/MS response per mole for the product derived from our new I2S substrate (6). The structure of our new GALNS substrate, GalNAc-6-S-C6/C6-benzoyl group (Bz), is shown in Fig. 1. The name derives from the presence of the N-hexanoyl group (C6), the hexamethylene linker (C6), and the Bz in the aglycone. Calculations suggest that the 2 carbonyl groups in the aglycone serve as a site of facile protonation (Fig. 1), which allows for higher-yield ionization in the electrospray source (11). We also pursued new assays for ARSB using GalNAc-4-S-C5/C5-Bz (Fig. 1).
Fig. 1. Structures of the substrates for assaying GALNS, ARSB, and I2S.
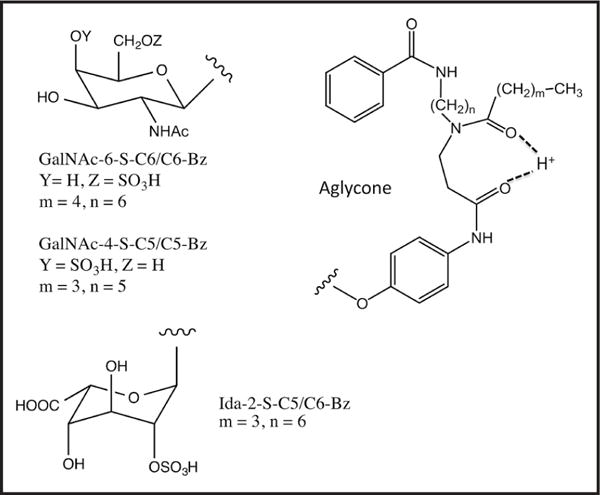
The proton shown hydrogen bonded to the 2 carbonyl groups is the proposed site of gas phase protonation in the electrospray ion source. Internal standards lack the sulfate and contain 5 deuteriums on the benzoyl group.
Online Supplemental Table 3 shows that the kcat/Km for GalNAc-6-S-C6/C6-Bz was 81-fold larger than that for Gal-6-S-C7/C6-Bz, indicating that GALNS greatly preferred the GalNAc substrate (we did not have the comparator compound Gal-6-S-C6/C6-Bz, but it is unlikely that GalNAc distinguishes between the number of methylenes in the aglycone). These studies justified the selection of our new GALNS GalNAc-6-S-C6/C6-Bz for continued studies.
The first version of the MS/MS assay was based on flow injection analysis (FIA). We considered the possibility of adding an enzyme that cleaved the glycoside only after the sulfatase removed the sulfate, thus generating the aglycone (Fig. 2). If the aglycone product gave a stronger MS/MS response per mole than that for the immediate sulfatase product (desulfated glycoside), use of the coupled enzymatic process would yield a gain in analytical range. Furthermore, buffer salt removal by liquid–liquid extraction was required for FIA-MS/MS to prevent suppression during the ionization process. In the case of sulfatases the initial product contains the sugar, which is hydrophilic and reduced the solvent extraction yield. Conversion to the aglycone will increase the transfer of analyte to the organic layer.
Fig. 2. Enzymatic reactions for I2S, GALNS, and ARSB.
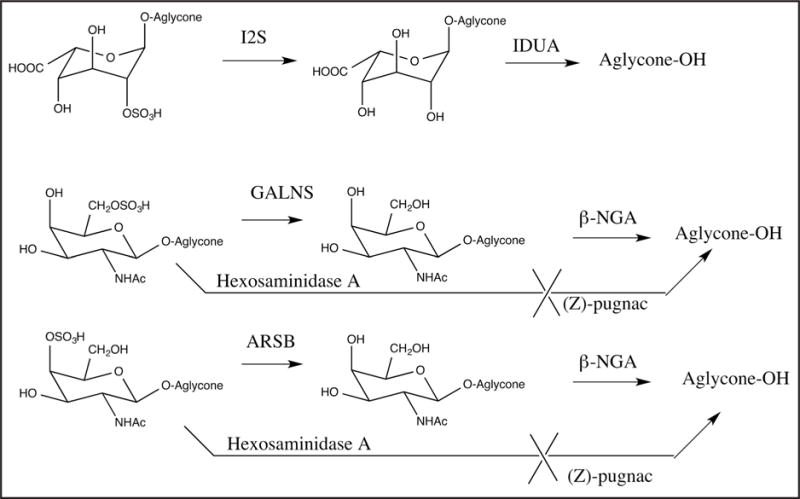
Coupling enzymes to convert the direct product of the lysosomal enzyme to the free aglycone are shown (this step is omitted for some variations of the assays described in the main text). The use of (Z)-Pugnac to block the action of human hexosaminidase A on the GALNS and ARSB substrates is also shown.
The ion response per mole of the GALNS- and ARSB-generated aglycones, C6/C6-Bz and C5/C5-Bz, were both found to be 2.4-fold larger than that for the corresponding glycosides GalNAc-6-S-C6/C6-Bz and GalNAc-4-S-C5/C5-Bz. This finding, along with the ethyl acetate consideration, argued for use of a coupled assay with enzymatic removal of the desulfated sugar. A disadvantage of this approach became apparent when we studied the pH dependence of the nonenzymatic hydrolysis of GalNAc-6-sulfate-C6/C6-Bz to the aglycone (see online Supplemental Fig. 1). Glycoside hydrolysis in the absence of DBS increased with a drop in pH, showing that it was acid catalyzed. It is known that glycosides of 2-acetamido sugars are more acid sensitive than those with a 2-hydroxyl because of neighboring group participation by the acetamido group (12). This acid-catalyzed pathway gave rise to aglycone, thus increasing the background of the assay. With DBS, enzymatic formation of the sulfatase product GalNAc-C6/C6-Bz was maximal at pH approximately 5.0, and thus going to pH >5.0 would not help. Similar pH-dependent observations were seen with the ARSB substrate GalNAc-4-S-C5/C5-Bz (see online Supplemental Fig. 1).
The use of GalNAc-4-S-C5/C5-Bz and GalNAc-6-S-C6/C6 required complete inhibition of hexosaminidase A because this enzyme can cleave the glycoside of GalNAc residues containing a 6-sulfate (13) (Fig. 2; also see online Supplemental Table 4). Recombinant human hexosaminidase A cleaved GalNAc-4-S-C5/C5-Bz, showing that the enzyme tolerated 4-sulfation (see online Supplemental Table 5). Endogenous hexosaminidase A present in DBS cleaved these glycosides (see online Supplemental Table 5). The hexosaminidase A inhibitor (Z)-Pugnac (14) at 1 mmol/L blocked almost all of the activity of recombinant hexosaminidase A and enzyme in DBS (see online Supplemental Table 6). In subsequent assays we used 2 mmol/L (Z)-Pugnac to ensure complete hexosaminidase A inhibition. To generate the aglycone from the desulfated glycoside, we explored the bacterial enzyme β-N-acetylgalactosaminidase (β-NGA) (15). The data in online Supplemental Tables 6 and 7 show that β-NGA did not generate aglycones from the sulfated substrates, and β-NGA catalyzed hydrolysis of the non-sulfated GALNS and ARSB products were not sensitive to (Z)-Pugnac. This allowed us to develop the new FIA-MS/MS assay of GALNS and ARSB based on the natural GalNAc-4/6-sulfate substrates (described above).
The internal standard for GALNS is GalNAc-C6/C6-Bz with 5 deuteriums in the benzoyl group. Thus, any incomplete β-NGA reaction is accounted for by using the glycoside internal standard. We found that increasing the amount of β-NGA beyond that in our standard assays did not increase the overall rate of aglycone formation (data not shown), showing that the reaction was limited by the sulfatase as desired. Selective reaction monitoring was used with FIA-MS/MS to measure the amount of nondeuterated product aglycone and deuterated internal standard aglycone after collision-induced dissociation of precursor ions.
Table 1 lists the analytical range of the FIA-MS/MS assays for GALNS and ARSB. The analytical range was defined as the ratio of assay response for the QC high sample due to the relevant enzyme divided by the response for nonenzymatic processes (calculated as described in the online Data Supplement).
Table 1.
Performance of the MS/MS assays of GALNS, ARSB, and I2S.
| Assay | MS/MS product response (peak area) | Analytical rangea | Mean (%CV) activity for 5 replicates of the QC DBS, μmol · h−1 · L−1a |
|---|---|---|---|
| GALNS FIA-MS/MS with β-NGA | Filter paper, 17 340 PE QC H, 220 050 |
23.4 (PE QC H) | No data |
| GALNS LC-MS/MS without β-NGA | Filter paper, 525 CDC QC H, 138 460 PE QC H, 230 905 |
120 (CDC QC H) 198 (PE QC H) |
Filter paper, 0.02 (9.1%) CDC QC B, 0.08 (1.8%) CDC QC L, 0.22 (8.7%) CDC QC M, 1.36 (12.8%) CDC QC H, 2.46 (12.3%) PE QC L, 0.29 (19.0%) PE QC M, 2.70 (12.0%) PE QC H, 4.07 (7.2%) |
| ARSB FIA-MS/MS with β-NGA | Filter paper, 20 520 PE QC H, 360 020 |
22.4 (PE QC H) | No data |
| ARSB LC-MS/MS without β-NGA | Filter paper, 2750 CDC QC H, 1 209 484 PE QC H, 1 406 271 |
170 (CDC QC H) 226 (PE QC H) |
Filter paper, 0.12 (6.1%) CDC QC B, 0.22 (3.6%) CDC QC L, 1.02 (10.2%) CDC QC M, 11.76 (6.4%) CDC QC H, 20.51 (11.2%) PE QC L, 1.96 (9.5%) PE QC M, 18.11 (5.7%) PE QC H, 27.39 (3.7%) |
| I2S FIA-MS/MS with IDUA | Filter paper, 9902 CDC QC H, 1 379 295 PE QC H, 3 297 559 |
257 (CDC QC H) 387 (PE QC H) |
Filter paper, 0.05 (63.9%) CDC QC B, 1.05 (6.2%) CDC QC L, 1.72 (4.8%) CDC QC M, 8.50 (7.2%) CDC QC H, 11.64 (6.1%) PE QC L, 1.17 (3.8%) PE QC M, 9.90 (3.5%) PE QC H, 17.50 (2.8%) |
| I2S LC-MS/MS with IDUA | Filter paper, 4146 CDC QC H, 2 207 739 PE QC H, 3 104 262 |
424 (CDC QC H) 721 (PE QC H) |
Filter paper, 0.03 (60%) CDC QC B, 1.31 (6.3%) CDC QC L, 2.19 (4.3%) CDC QC M, 10.35 (2.7%) CDC QC H, 12.93 (6.4%) PE QC L, 1.34 (7.0%) PE QC M, 11.42 (5.8%) PE QC H, 19.40 (3.3%) |
| I2S LC-MS/MS without IDUA | Filter paper, 1756 CDC QC H, 1 578 629 PE QC H, 1 905 935 |
521 (CDC QC H) 681 (PE QC H) |
Filter paper, 0.04 (145.7%) CDC QC B, 1.10 (6.4%) CDC QC L, 2.20 (3.6%) CDC QC M, 12.60 (4.7%) CDC QC H, 18.66 (5.6%) PE QC L, 1.53 (2.2%) PE QC M, 14.29 (3.1%) PE QC H, 24.41 (6.4%) |
Values calculated as described in the online Data Supplement. QC samples from the CDC are base pool (B, 0% whole blood), low (L, 5% whole blood), medium (M, 50% whole blood), and high (H, 100% whole blood). Same for the PerkinElmer (PE) samples except there is no base pool.
LC-MS/MS ASSAYS FOR GALNS AND ARSB
Given the multiple factors that contribute to analytical range in the FIA-MS/MS assay using β-NGA, we also studied a second assay for GALNS and ARSB based on coupled LC-MS/MS. In this case, sample desalting occurred on the liquid chromatography (LC) column, and thus conversion of the glycoside product to the aglycone with β-NGA and liquid–liquid extraction with ethyl acetate were not required. The assay incubation was quenched with acetonitrile, and precipitated macromolecules were pelleted by centrifugation. The supernatant was diluted with water and subjected to LC-MS/MS. Because we detected the desulfated products GalNAc-C5/C5-Bz and GalNAc-C6/C6-Bz rather than the aglycones, we were not concerned with acid-catalyzed glycoside hydrolysis. We were also not concerned with enzyme-independent desulfation of substrates in the electrospray ion source because substrate and product had distinct LC retention times (see online Supplemental Fig. 2), and we integrated only the region of the chromatograph in which product and internal standard eluted. (Z)-Pugnac was still added because a substantial amount of the internal standard was cleaved without the hexosaminidase A inhibitor (not shown). To minimize the instrumental complexity, we used a normal-pressure LC column and developed an isocratic solvent system to separate the GALNS and ARSB products from their corresponding substrates. In this way we simply inserted the LC column into the solvent delivery system that was used for FIA-MS/MS. Furthermore, in our previous studies of LC-MS/MS for LSD assays (16), several thousand assays could be obtained without column replacement.
Table 1 lists the analytical ranges for the GALNS and ARSB LC-MS/MS assays. Given the higher analytical ranges, we carried out additional studies using LC-MS/MS rather than FIA-MS/MS assays. To demonstrate the reproducibility of the assay and the linearity with respect to the amount of enzyme in the DBS, we carried out 5 independent replicate assays on a series of QC DBS containing various amounts of leukocyte-depleted and whole blood (Table 1; linearity plots provided in online Supplemental Fig. 3).
Assay results for 50 random newborns and MPS-IVA– and MPS-VI–affected patients are provided in Fig. 3 (tabular data in online Supplemental Tables 8 and 9), showing that this LC-MS/MS assay readily distinguished between 50 healthy newborns and MPS-IVA or MPS-VI patients.
Fig. 3. Enzyme activities in random newborns and LSD-affected patients measured using LC-MS/MS.
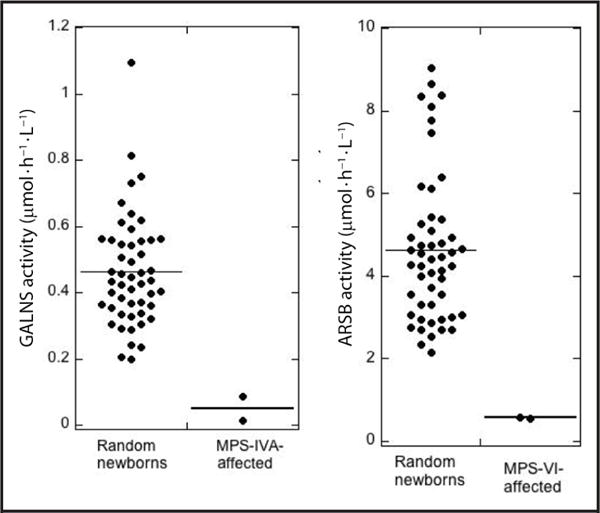
Note the difference in y-axis scales. Mean activity is shown as a horizontal line.
MS/MS ASSAYS FOR I2S
We explored a variation of our previous MS/MS assay for I2S by adding IDUA to generate the aglycone after desulfation (Fig. 2). We found that the glycoside IdA-C5/C6-Bz gave an approximately 5-fold lower MS/MS response per mole than did its aglycone (not shown). We carried out 3 assay variants: (a) coupled assay with IDUA, ethyl acetate extraction, FIA-MS/MS; (b) IDUA, protein precipitation with acetonitrile, LC-MS/MS; and (c) no IDUA, acetonitrile, LC-MS/MS. Table 1 summarizes the results with QC DBS. All 3 methods gave similar analytical ranges. LC traces and linearity data are shown in online Supplemental Figs. 2 and 3.
TRIPLEX ASSAY FOR MPS-II, -IVA, AND -VI
After exploring several possible options, we put all 3 MPS assays into a single triplex assay using a single cocktail incubated with a single 3-plex DBS punch. For solubility reasons we reduced the concentration of the MPS-II substrate to 0.5 mmol/L and kept the other 2 substrates at 1 mmol/L (I2S had the highest activity). We included citric acid in the quench step so that the MPS-II product containing the iduronic acid group would protonate on its carboxylate to facilitate extraction into ethyl acetate during the liquid–liquid extraction. The extract was submitted to LC-MS/MS using the above isobaric conditions under low pressure. Ethyl acetate extraction was found to provide a more robust assay because it led to a large reduction in the amount of substrate in the mixture applied to the LC column, which in turn reproducibly ensured complete separation between substrates and product/internal standards. Results are summarized in Table 2, and linearity data using QC standards is shown in online Supplemental Fig. 3.
Table 2.
Performance of the triplex assay for I2S, GALNS, and ARSB.
| Enzyme | MS/MS product response (peak area) | Analytical rangea | Mean (%CV) activity for 5 replicates of the QC DBS, μmol · h−1 · L−1a |
|---|---|---|---|
| I2S | Filter paper, 4100 | 430 (CDC QC H) | Filter paper, 0.028 (5.0%) |
| CDC QC H, 2 151 806 | 577 (PE QC H) | CDC QC B, 0.732 (5.7%) | |
| PE QC H, 2 737 395 | CDC QC L, 1.50 (5.1%) | ||
| CDC QC M, 8.49 (5.7%) | |||
| CDC QC H, 11.93 (11.3%) | |||
| PE QC L, 1.19 (8.2%) | |||
| PE QC M, 10.22 (2.5%) | |||
| PE QC H, 16.01 (1.7%) | |||
| GALNS | Filter paper, 3096 | 85 (CDC QC H) | Filter paper, 0.024 (4.7%) |
| CDC QC H, 248 713 | 119 (PE QC H) | CDC QC B, 0.077 (3.7%) | |
| PE QC H, 353 333 | CDC QC L, 0.203 (9.4%) | ||
| CDC QC M, 1.26 (8.3%) | |||
| CDC QC H, 2.05 (10.3%) | |||
| PE QC L, 0.236 (7.7%) | |||
| PE QC M, 2.10 (3.2%) | |||
| PE QC H, 1.86 (5.8%) | |||
| ARSB | Filter paper, 3265 | 143 (CDC QC H) | Filter paper, 0.058 (4.5%) |
| CDC QC H, 453 074 | 188 (PE QC H) | CDC QC B, 0.094 (5.9%) | |
| PE QC H, 584 751 | CDC QC L, 0.47 (9.3%) | ||
| CDC QC M, 5.34 (8.1%) | |||
| CDC QC H, 8.21 (14.0%) | |||
| PE QC L, 0.90 (8.8%) | |||
| PE QC M, 7.90 (3.2%) | |||
| PE QC H, 10.83 (6.6%) |
Values calculated as described in the online Data Supplement. QC samples from the CDC are base pool (B, 0% whole blood), low (L, 5% whole blood), medium (M, 50% whole blood), and high (H, 100% whole blood). Same for the PerkinElmer (PE) samples except there is no base pool.
ANALYTICAL RANGE FOR FLUOROMETRIC ENZYME ASSAYS BASED ON 4MU SUBSTRATES
We used the 4MU substrates in Table 3 to assay lysosomal enzymes IDUA, acid α-glucosidase, I2S, GALNS, and ARSB. The principle of these assays is based on enzymatic release of 4MU from the substrate conjugate (i.e., 4MU-glycosides). The mixture was quenched with a buffer with a pH of approximately 10 to cause ionization of the hydroxyl group of 4MU to its phenolate (4MU-OH to 4MU-O−). The pKa of 4MU-OH is 7.6, and the 4MU-O−/4MU-OH fluorescence emission ratio is approximately 16 (17). We confirmed these findings by spectral titration of 4MU (not shown). The above analysis showed that the neutral species 4MU-OH had finite fluorescence, and thus we explored the fluorescence of 4MU-glycoside substrates. Table 3 lists the percentages of 4MU present as an impurity in the substrates and the ratio (4MU-O− emission per mole)/(4MU-glycoside emission per mole). These ratios were in the range 2200–5400 (the outlier was for 4MU-sulfate, ratio = 58 700) and accounted for the increase in fluorescence when 4MU-glycosides were acted on by lysosomal enzymes. Although this ratio was much higher than 1, in assays with DBS, the percentage of total 4MU-substrate that was converted to 4MU was typically only approximately 1%. Thus, the intrinsic fluorescence of the 4MU-glycoside substrate greatly increased the background and reduced the analytical range.
Table 3.
Fluorimetry assays with 4MU glycosides.a
| 4MU-glycoside/enzyme | Amount of 4MU contaminant (mol %) | 4MU-O−/4MU-glycoside emission ratio | Analytical range | Enzyme activity, μmol · h−1 · L−1 | Percent substrate converted to product |
|---|---|---|---|---|---|
| 4MU-α-L-iduronide/IDUA | 0.067 | 2744 | 16.3 (CDC High) 12.3 (PE High) |
12.5 (CDC High) 9.6 (PE High) |
2.1 (CDC High) 1.6 (PE High) |
| 4MU-α-D-glucose/acid α-glucosidase |
0.023 | 2245 | 16.6 (CDC High) 12.3 (PE High) |
17.5 (CDC High) 10.1 (PE High) |
2.9 (CDC High) 1.7 (PE High) |
| 4MU-α-L-iduronide-2-sulfate/I2S 4MU-sulfate/ARSB |
0.025 0.032 |
2520 58 700 |
11.4 (PE High) 34.0 (PE High) |
8.7 (PE High) 5.0 (PE High) |
1.4 (PE High) 0.16 (PE High) |
| 4MU-β-N-GalNAc-4-sulfate/ARSB | 0.010 | 5390 | 7.8 (PE High) | 5.94 (PE High) | 0.33 (PE High) |
| 4MU-β-N-GalNAc-6-sulfate/GALNS | 0.016 | 4120 | 6.5 (PE High) | 9.14 (PE High) | 0.51 (PE High) |
Values calculated as described in the online Data Supplement. PE, PerkinElmer.
The analytical ranges of these 4MU assays were defined as above (details of the calculation provided in the online Data Supplement) and listed in Table 3. Background-corrected enzyme activities are also listed in Table 3.
Discussion
ANALYTICAL RANGE FOR 4MU-FLUOROMETRIC VS MS/MS ASSAYS OF LYSOSOMAL ENZYMES
For the 6 4MU assays studied, the analytical ranges were in the range 6–16 (except 34 for ARSB with 4MU-sulfate) (Table 3) when measured with the same QC high DBS (PerkinElmer QC High, see Methods in the online Data Supplement). For all of the assays shown in Table 2, the fluorescence from the buffer or the buffer incubated with the DBS punch, both lacking substrate, was <5% of the fluorescence measured with buffer containing 4MU-glycoside substrate (data not shown). What limits the analytical range of these assays is clearly the high background due to the intrinsic fluorescence of the 4MU-glycoside substrates and the small fraction of substrate converted to product.
The analytical ranges for the MS/MS assays were much larger than those for 4MU assays, 51-fold for I2S and 30-fold for GALNS. For ARSB, the MS/MS analytical range was 6.6-fold higher than that for the 4MU assay if 4MU-sulfate was used or 29-fold if the more natural-like substrate 4MU-GalNAc-4-S was used (see below for concerns about using 4MU-sulfate). We hypothesize that the assays with higher analytical range will lead to a better separation between nonaffected and LSD-affected individuals, which will lead to lower false-positive rates in NBS and to more accurate estimation of disease prognoses. Comparison of large-scale NBS pilot studies has already shown evidence that the MS/MS method leads to substantially lower false positives (18–20).
GALNS ASSAYS
The originally reported assay for GALNS used 4MU-Gal-6-sulfate (21) and leukocytes as the enzyme source. This assay relied on endogenous human β-galactosidase to release the 4MU after desulfation by GALNS. Extension of this assay to DBS involved supplementation of the cocktail with Apergillus oryzae β-galactosidase (Sigma Chemicals) that is active at pH approximately 4 (7). We attempted this GALNS assay with DBS and found that the commercial Apergillus oryzae β-galactosidase from Sigma was capable of generating 4MU from 4MU-Gal-6-sulfate (no difference in assay response was observed for assays with DBS vs filter paper, data not shown), suggesting that this preparation of enzyme is contaminated with a 6-sufatase. The calculation of the analytical range of this GALNS assay with DBS was not possible because the assay responses for the blanks were not stated (7). However, we can say that the mean GALNS activity measured for 50 random newborn DBS with 3 mmol/L 4MU-GalNAc-6-S is 3.8 μmol · h−1 · L−1 (22) compared to 0.28 μmol · h−1 · L−1 reported using 10 mmol/L 4MU-Gal-6-S (7). This suggests that GALNS is approximately 44-fold more active on 4MU-GalNAc-6-S vs 4MU-Gal-6-S, which is consistent with our MS/MS data (see online Supplemental Table 3). Thus the analytical range using 4MU-Gal-6-S is likely less than the value of 6.5 for 4MU-GalNAc-6-S. These relatively low analytical ranges suggest that the 4MU assays for GALNS will not be appropriate for NBS of MPS-IVA. The enormous increase in analytical range for the LC-MS/MS assay vs the fluorometric assay for GALNS is due mainly to the factors described above that limit the analytical range of the 4MU assays rather than a difference in the enzyme activities (μmol · h−1 · L−1) for the MS/MS vs 4MU substrates (the same is true for the ARSB and I2S assays, see below).
The GALNS FIA-MS/MS method requires the use of β-NGA and ethyl acetate extraction. The analytical range is 4-fold better than that for the 4MU-GalNac-6-S fluorometric assay (Tables 1 and 2), and the LC-MS/MS assay in the absence of β-NGA and ethyl acetate is 8.5-fold better than the FIA-MS/MS assay and provides a high-performance GALNS assay (Table 2). Product and ion counts are approximately 200-fold higher than those of our original GALNS MS/MS assay based on Gal-6-S (8). This assay readily distinguished between 50 healthy newborns and the MPS-IVA patients (Fig. 3). Pilot studies using this new GALNS assay (and ARSB assay, see below) have started in the Washington State Department of Health NBS laboratory, which seems timely given that enzyme replacement treatment is now available (23). Adding a low-pressure LC column with an isocratic elution solvent does not add significant instrumentation complexity because the low pressure column is compatible with currently used FIA solvent delivery systems and column cost is insignificant.
ARSB ASSAYS
The established assay for ARSB for diagnosis of MPS-VI uses the nonspecific sulfatase substrate 4MU-sulfate or related catechol sulfates (24). Studies with DBS from MPS-VI patients show low but residual ARSB activity, suggesting that this substrate is detecting mainly ARSB. The source of this residual activity is unknown. It may be due to residual activity of ARSB or due to other sulfatases. Of interest is arylsulfatase A, the enzyme deficient in metachromatic leukodystrophy, because it hydrolyzes 4MU-sulfate (4, 25). Arylsulfatase A is active in samples using DBS because it can be detected after immunoprecipitation and subsequent assay with 4MU-sulfate (25). Additional studies show that this enzyme is relatively unstable in DBS (25). All together the studies strongly suggest that the use of the nonspecific sulfatase substrate 4MU-sulfate is not appropriate for NBS of MPS-VI in which fresh DBS will be the enzyme source. Caution is also warranted for diagnostic assays using DBS or leukocyte samples that have not been confirmed to lack arysulfatase A activity. The distinction between ARSB and arylsulfatase A using 4MU-sulfate can be improved by using different buffer conditions for the 2 assays (4), but there is still considerable crosstalk between these 2 assays (4).
The ARSB FIA-MS/MS assay described here uses β-NGA, hexosaminidase A inhibitor, and ethyl acetate extraction. The analytic range is comparable to the fluorometric assays with 4MU-sulfate and 3-fold better than the fluorometric assay with 4MU-GalNAc-4-S. Again, the LC-MS/MS assay without coupling enzyme is superior, being 10-fold better in analytical range than the FIA-MS/MS assay. The assay distinguishes healthy newborns from MPS-VI patients (Fig. 3).
I2S ASSAYS
The analytic range of the FIA-MS/MS assay is 34-fold higher than that with the 4MU-IdA-2 assay and is improved another approximately 2-fold if we use LC-MS/MS without the need for adding IDUA-coupling enzyme. A pilot study using the MS/MS I2S assay for NBS of MPS-II has begun in the Washington State Department of Health NBS laboratory.
TRIPLEX ASSAYS
Combining all 3 sulfatase assays into a single triplex assay using a single assay cocktail and a single 3-mm DBS punch was straightforward, and results shown in Table 2 and online Supplemental Fig. 3 demonstrate that the method works essentially as well as for the individual assays, again with analytic ranges that greatly exceed those seen with 4MU assays.
Supplementary Material
Acknowledgments
Special thanks to Nicole Miller (BioMarin) and Jason Cournoyer and Mack Schermer (PerkinElmer Life Sciences) for helpful discussions and to Dr. Christiane Auray-Blais (Univ. of Sherbrooke) for providing DBS.
Employment or Leadership: F. Turecek, University of Washington.
Consultant or Advisory Role: R. Scott, Genzyme Corp; M.H. Gelb, PerkinElmer.
Stock Ownership: None declared.
Honoraria: R. Scott, Genzyme Corp.
Research Funding: Schire Corp and research contract from BioMarin Pharmaceuticals; S. Masi, National Institutes of Health (R01 DK067859).
Expert Testimony: None declared.
Patents: Z. Spacil, patent number WO/2013/070953.
Footnotes
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest.
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or preparation or approval of manuscript.
References
- 1.Boustany RM. Lysosomal storage diseases-the horizon expands. Nature Rev Neurol. 2013;9:583–98. doi: 10.1038/nrneurol.2013.163. [DOI] [PubMed] [Google Scholar]
- 2.Chamoles NA, Blanco M, Gaggioli D. Fabry disease: enzymatic diagnosis in dried blood spots on filter paper. Clin Chim Acta. 2001;308:195–6. doi: 10.1016/s0009-8981(01)00478-8. [DOI] [PubMed] [Google Scholar]
- 3.Gelb MH, Scott CR, Turecek F. Newborn screening for lysosomal storage diseases. Clin Chem. 2014;61:335–46. doi: 10.1373/clinchem.2014.225771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular basis of inherited disease. 8th. New York: McGraw-Hill; 2001. [Google Scholar]
- 5.Voznyi YV, Keulemans JL, van Diggelen OP. A fluorometric assay for diagnosis of MPS II (Hunter disease) J Inherit Metab Dis. 2001;24:675–80. doi: 10.1023/a:1012763026526. [DOI] [PubMed] [Google Scholar]
- 6.Chennamaneni NK, Kumar AB, Barcenas M, Spacil Z, Scott CR, Turecek F, Scott CR. Improved reagents for newborn screening of mucopolysaccharidosis types I, II, and VI by tandem mass spectrometry. Anal Chem. 2014;86:4508–14. doi: 10.1021/ac5004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camelier MV, Burin MG, de Mari J, Vieira TA, Marasca G, Giugliana R. Practical and reliable enzyme test for the detection of mucopolysaccharidosis IVA (Morquio Syndrome type A) in dried blood samples. Clin Chim Acta. 2011;412:1805–8. doi: 10.1016/j.cca.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Khaliq T, Sadilek M, Scott CR, Turecek F, Gelb MH. Tandem mass spectrometry for the direct assay of lysosomal enzymes in dried blood spots: application to screening newborns for mucopolysaccharidosis IVA. Clin Chem. 2011;57:128–31. doi: 10.1373/clinchem.2010.149880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Civallero G, Michelin K, de Mari J, Viapiana M, Burin M, Coelho JC, Giugliani R. Twelve different enzyme assays on dried-blood filter samples for detection of patients with selected inherited lysososmal storage diseases. Clin Chim Acta. 2006;372:98–102. doi: 10.1016/j.cca.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 10.Duffey TA, Sadilek M, Scott CR, Turecek F, Gelb MH. Tandem mass spectrometry for the direct assay of lysosomal enzymes in dried blood spots: application to screening newborns for mucopolysaccharidosis VI (Maroteaux-Lamy syndrome) Anal Chem. 2010;82:9587–91. doi: 10.1021/ac102090v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spacil Z, Hui R, Gelb MH, Turecek F. Protonation sites and dissociation mechanisms of t-butylcarbamates in tandem mass spectrometric assays for newborn screening. J Mass Spectrom. 2011;46:1089–98. doi: 10.1002/jms.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jennings HJ, Lugowski C. Facile cleavage of some 2-acetamido-2-deoxy-β-D-gluco- and galactopyranosides using aqueous HF. Can J Chem. 1980;58:2610–2. [Google Scholar]
- 13.Bayleran J, Hechtman P, Saray W. Synthesis of 4-methylumbelliferyl-beta-D-n-acetylglucosamine-6-sulfate and its use in classification of GM2 gangliosidosis genotypes. Clin Chim Acta. 1984;143:73–89. doi: 10.1016/0009-8981(84)90215-8. [DOI] [PubMed] [Google Scholar]
- 14.Kim EJ, Perreira M, Thomas CJ, Hanover JA. An O-GlcNAcase-specific inhibitor and substrate engineered by the extension of the N-acetyl moiety. J Am Chem Soc. 2006;128:4234–5. doi: 10.1021/ja0582915. [DOI] [PubMed] [Google Scholar]
- 15.Sumida T, Fujimoto K, Ito M. Molecular cloning and catalytic mechanism of a novel glycosphingolipid-degrading beta-N-acetylgalactosaminidase from Paenibacillus sp. Ts12. J Biol Chem. 2011;286:14065–72. doi: 10.1074/jbc.M110.182592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spacil Z, Tatipaka H, Marcenas M, Scott CR, Turecek F, Gelb MH. High throughput assay of nine lysosomal enzymes for newborn screening. Clin Chem. 2013;59:502–11. doi: 10.1373/clinchem.2012.189936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen RF. Fluorescent pH indicator. Spectral changes of 4-methylumbellferone. Anal Lett. 1968;1:423–8. [Google Scholar]
- 18.Mechtler TP, Stary S, Metz TF, De Jesus VR, Greber-Platzer S, Pollak A, et al. Neonatal screening for lysosomal storage disorders: feasibility and incidence from a nationwide study in Austria. Lancet. 2012:335–41. doi: 10.1016/S0140-6736(11)61266-X. [DOI] [PubMed] [Google Scholar]
- 19.Scott CR, Elliott S, Buroker N, Thomas LI, Keutzer J, Glass M, et al. Identification of infants at risk for developing Fabry, Pompe, or mucopolysaccharidosis-I from newborn blood spots by tandem mass spectrometry. J Pediatr. 2013;163:498–503. doi: 10.1016/j.jpeds.2013.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hopkins P. Missouri’s experience with full population pilot screening for Pompe, Gaucher, Fabry and Hurler disorders using digital microfluidics methodology. 2014 Newborn Screening and Genetic Testing Symposium; 2014 Oct 27–30; Anaheim, CA. http://www.aphl.org/conferences/2014_newborn_screening_and_genetic_testing_symposium/pages/default.aspx (Accessed March 2015) [Google Scholar]
- 21.van Diggelen OP, Zhao H, Kleijer WJ, Janse HC, Poorthuis BJ, van Pelt J, et al. A fluorimetric enzyme assay for the diagnosis of Morquio disease type A (MPS IV A) Clin Chim Acta. 1990;187:131–9. doi: 10.1016/0009-8981(90)90339-t. [DOI] [PubMed] [Google Scholar]
- 22.Kumar AB, Spacil Z, Ghomashchi F, Masi S, Turecek F, Sumida T, et al. Fluorimetric assays forN-acetylgalactosamine-6-sulfatase and arylsulfatase B based on the natural substrates for confirmation of mucopolysaccharidoses types IVA and VI. Clin Chim Acta. doi: 10.1016/j.cca.2015.08.010. [Epub ahead of print 2015 Aug 15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schweighardt B, Tompkins T, Lau K, Jesaitis L, Qi Y, Musson DG, et al. Immunogenicity of elosulfase alfa, an enzyme replacement therapy in patients with Morquio A syndrome: results from mor-004, a phase III trial. Clin Ther. 2015;37:1012–21. doi: 10.1016/j.clinthera.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Ullal AJ, Millington DS, Bali DS. Development of a fluorometric microtiter plate-based enzyme assay for arylsulfatase B (MPS VI) using dried blood spots. Mol Genet Metab Rep. 2014;1:465–7. doi: 10.1016/j.ymgmr.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan MA, Dean CJ, Hopwood JJ, Meikle PJ. Diagnosis of metachromatic leukodystrophy by immune quantification of arylsulphatase A protein and activity in dried blood spots. Clin Chem. 2008;54:1925–7. doi: 10.1373/clinchem.2008.108456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


