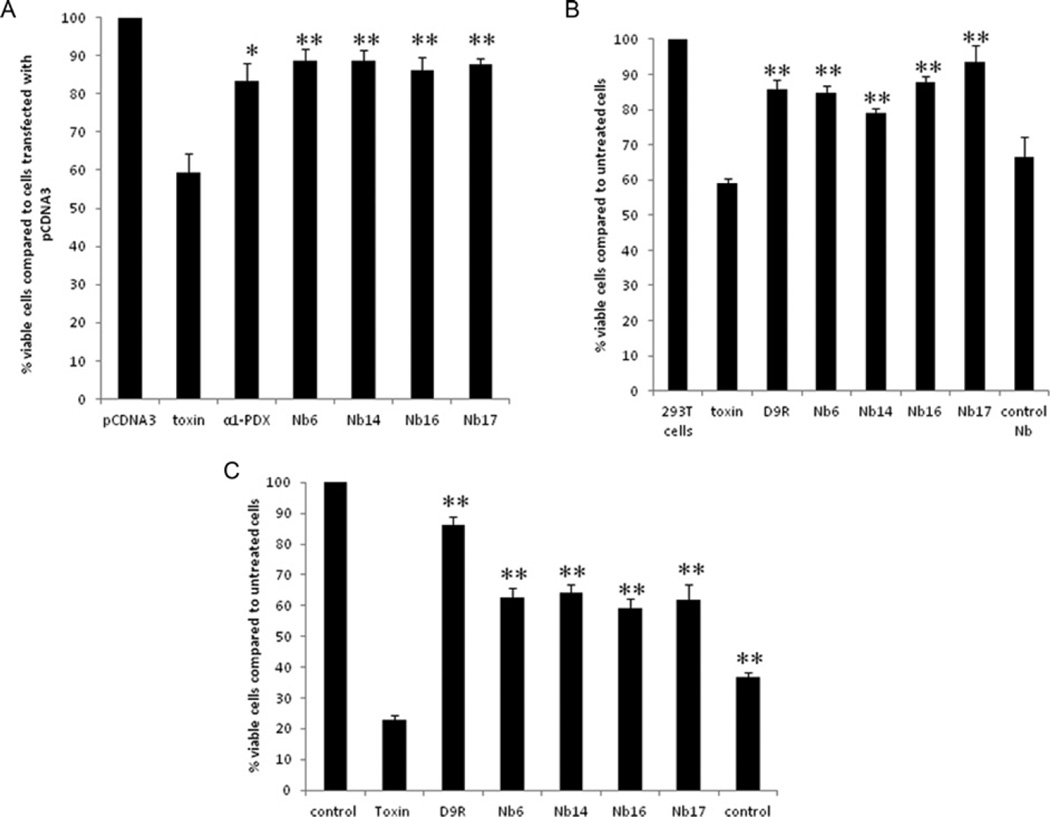
Figure 5. The nanobodies inhibit diphtheria-toxin-mediated cytotoxicity as efficiently as α1-PDX and D9R.
(A) HEK-293T cells were transfected with empty vector, an expression vector encoding α1-PDX or the different nanobodies 24 h before exposure to diphtheria toxin. Then, 3 h after adding diphtheria toxin, cell viability was assessed by the MTT assay. The nanobodies protected the cells from cytotoxicity as efficiently as the well-characterized furin inhibitor α1-PDX. The viability of cells treated with the diphtheria toxin together with α1-PDX or the different nanobodies is significantly higher when compared with cells only treated with the diphtheria toxin. (B) At 2 h prior to the exposure to diphtheria toxin, HEK-293T cells were incubated with 10 µM of purified nanobodies or with D9R. Then 1.5 h after adding the diphtheria toxin, cell viability was assessed by the MTT assay. The nanobodies targeting furin significantly protected the cells from cytotoxicity; protection was as efficient as the well-characterized furin inhibitor D9R, whereas control nanobodies which do not target furin did not inhibit cell toxicity. (C) At 30 min prior to the exposure to anthrax toxin (200 ng/ml PA and 400 ng/ml LF), RAW cells were incubated with 20 µM purified nanobodies or with 10 µM D9R. Then, 1.5 h after adding the toxins cell viablility was assayed using the MTT assay. Nanobodies targeting furin significantly protected the cells from cytotoxicity although less efficient than D9R, whereas control nanobodies which do not target furin only showed a mild effect on the cell toxicity. Results are presented as means ± S.E.M. (n =3) *P ≤0.05, **P ≤0.005.