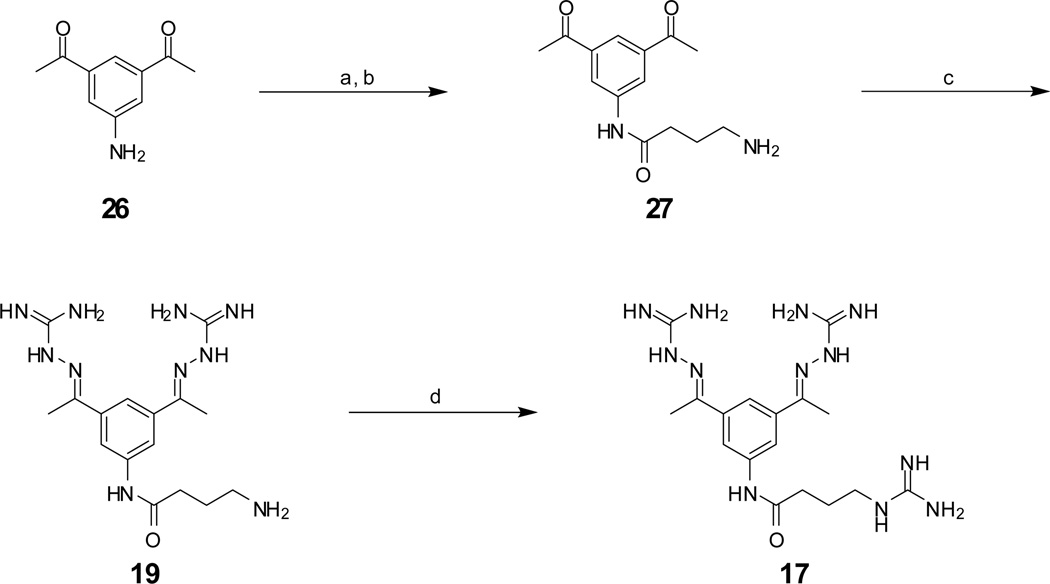
Scheme 1.
Reagents and conditions: (a) Boc-γ-aminobutyric acid, N-methylmorpholine, isobutylchloroformate, −15°C, 10 min in DMF, followed by addition of 26, 1h at −15°C and overnight at room temperature, (b) 1N HCl in acetic acid, 1h room temperature, (c) 2.6 equiv. aminoguanidine hydrochloride, 5 mol % HCl, in 50 % EtOH reflux for 6h, (d) 3 equiv. 1H-pyrazole-carboxamidine hydrochloride, 6 equiv. diisopropylethylamine in DMF, 16h. Final inhibitors 19 and 17 were purified by preparative reversed phase HPLC to a purity of >95% according to HPLC at 220 nm.