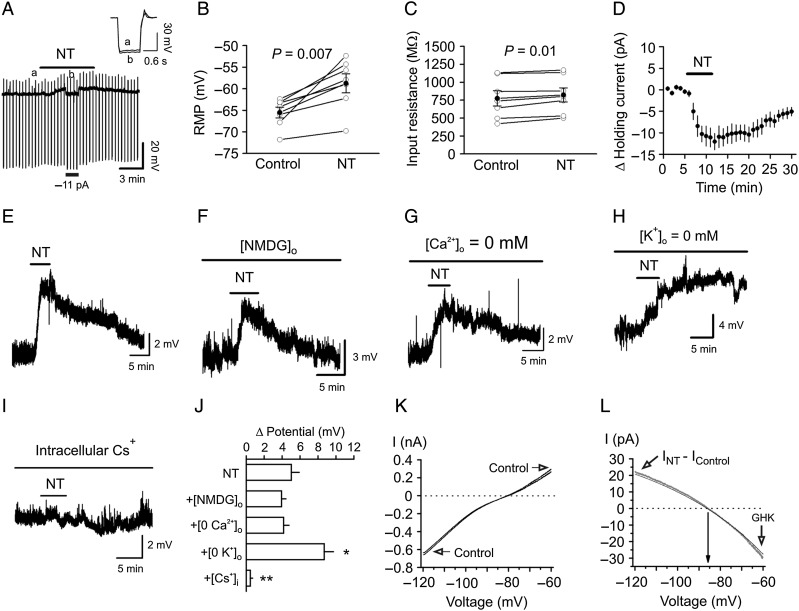
Figure 4.
NT-mediated enhancement of neuronal excitability is mediated by inhibiting a background K+ channel. (A) Bath application of NT generated membrane depolarization and slightly increased the input resistance of the GCs. RMP was recorded in current-clamp mode, and a hyperpolarizing current (−50 pA, 500 ms) was injected every 20 s to measure the input resistance. Note that NT generated depolarization and slightly increased the input resistance. To exclude the influence of NT-induced membrane depolarization on the input resistance, a negative current (−11 pA indicated by the horizontal bar) was injected briefly to bring the membrane potential back to the initial level. Inset is the voltage traces taken before (a) and during (b) the application of NT when the negative current was injected. (B) Pooled RMPs from 7 cells before and during the application of NT. (C) Pooled input resistance from 7 cells before and during the application of NT. (D) Application of NT induced an inward HC. (E) NT induced membrane depolarization recorded in current clamp from a GC. (F) Replacement of extracellular NaCl with NMDG-Cl did not block NT-induced depolarization. (G) Substitution of extracellular Ca2+ with Mg2+ and inclusion of EGTA (1 mm) in the extracellular solution failed to annul NT-induced depolarization. (H) NT induced an even larger depolarization in the absence of extracellular K+. (I) Bath application of NT failed to induce depolarization when Cs+-gluconate was included in the recording pipette. (J) Summary bar graph. *P < 0.05, **P < 0.01 vs. NT alone. (K) I–V curves recorded by a ramp protocol (from −120 to −60 mV) in the extracellular solution containing 3.5 mm K+ before and during the application of NT. (L) The NT-generated net current obtained by subtraction of the control from that in the presence of NT could be fit by GHK equation.