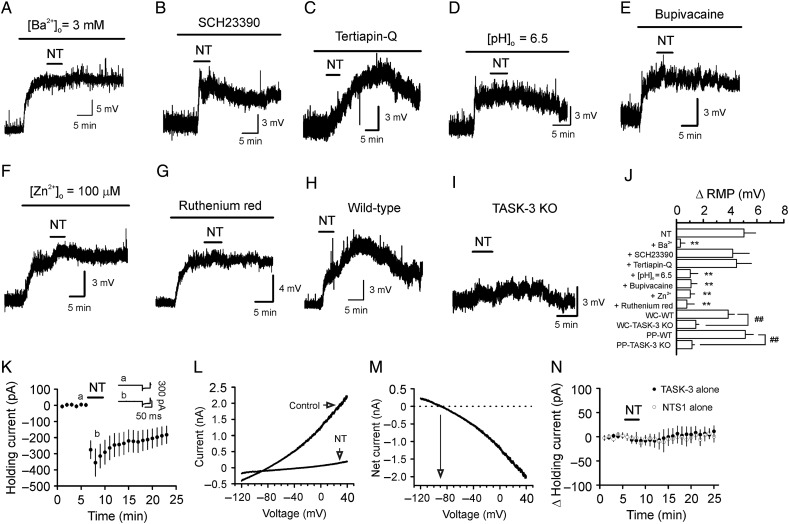
Figure 5.
NT depolarizes the GCs by inhibiting TASK-3 channels. (A) Bath application of Ba2+ by itself induced membrane depolarization, and subsequent application of NT failed to further alter the membrane potential. (B) Application of SCH23390 (20 µm), a Kir inhibitor, failed to block NT-induced depolarization. (C) Application of tertiapin-Q (0.5 µm), another Kir inhibitor, failed to block NT-induced depolarization. (D) Lowering the extracellular solution pH from 7.4 to 6.5 induced a depolarization and blocked NT-induced depolarization. (E) Application of bupivacaine (200 µm), a TASK channel blocker, induced depolarization of the GCs and blocked NT-induced depolarization. (F) Inclusion of Zn2+ (100 µm), a TASK-3 channel blocker, in the extracellular solution produced depolarization and reduced NT-induced depolarization. (G) Inclusion of ruthenium red (10 µm), another TASK-3 channel blocker, in the extracellular solution depolarized the GCs and blocked NT-induced depolarization. (H) Bath application of NT depolarized the GCs in slices cut from WT mice. (I) Bath application of NT induced significantly smaller level of depolarization of the GCs in slices cut from TASK-3 KO mice. (J) Summary bar graph. WC-WT: whole-cell recording from slices cut from WT mice; WC-TASK-3 KO: whole-cell recording from slices cut from TASK-3 KO mice; PP-WT: perforated-patch recording from slices cut from WT mice; PP-TASK-3 KO: perforated-patch recording from slices cut from TASK-3 KO mice. (K) NT inhibited TASK-3 currents in HEK293 cells coexpressing NTS1 receptors and TASK-3 channels. (L) I–V curves recorded from a HEK293 cell coexpressing NTS1 and TASK-3 before and during the application of NT. (M) I–V curve of the net current generated by subtraction of the I–V curve before from that in the presence of NT. (N) Bath application of NT did not generate significant inward HCs in HEK293 cells expressing only NTS1 or TASK-3. **P < 0.01 vs. NT alone; ##P < 0.01.