Abstract
Tourette syndrome (TS) is a neurodevelopmental disorder characterized by multiple chronic motor and vocal tics beginning in childhood. Several studies describe the association between TS and attention deficit hyperactivity disorder (ADHD). Fifty percent of children diagnosed with ADHD have comorbid tic disorder. ADHD related symptoms have been reported in 35% to 90% of children with TS. Since ADHD is the most prevalent comorbid condition with TS and those with concomitant TS and ADHD present with considerable psychosocial and behavioral impairments, it is essential for clinicians to be familiar with these diagnoses and their management. This paper highlights the association between treating ADHD with stimulants and the development of tic disorders. The two cases discussed underscore the fact that children with TS may present with ADHD symptomatology prior to the appearance of any TS related symptoms. Appropriate management of TS in a patient diagnosed with ADHD can lead to quality of life improvements and a reduction in psychosocial impairments.
Keywords: Tourette syndrome, Psychopharmacology, Attention deficit hyperactivity disorder, Tics
Core tip: Tics can be a symptom of Tourette syndrome (TS). Attention deficit hyperactivity disorder (ADHD) has the highest comorbidity with TS. Psychopharmacological treatment of ADHD with stimulants may cause, or exacerbate pre-existing, tics. Because of this, providers may be reluctant to use stimulants in patients with comorbid tic disorders. However, the role of stimulants in the treatment of TS associated with ADHD, when the benefits outweigh the risks, cannot be over emphasized as a comprehensive approach, considering all treatment options for managing TS and ADHD will yield better outcomes.
INTRODUCTION
Tourette syndrome (TS) is a neurodevelopmental disorder characterized by multiple chronic motor and vocal tics developing before adulthood[1]. Tics are sudden, rapid, recurrent, non-rhythmic, stereotyped and involuntary motor movements or vocalizations. They are classified as either simple or complex, with the former affecting several muscle groups. Motor tics can affect any part of the body with varying location, frequency and complexity of movements which may change over time. Vocal tics, also known as phonic tics, are involuntary sounds produced by air motion through the nose, mouth, or throat[1,2]. See Table 1 for types and description of tics[1,3].
Table 1.
Types and description of tics
| Simple | Complex | |
| Vocal or Phonic | Simple phonic/vocal tics: These are sudden meaningless noises or sounds | Complex phonic/vocal tics: These are sudden and more meaningful words, syllables or phrases |
| Examples: Throat clearing, coughing, spitting, barking, hissing, sucking, clacking, gurgling, sniffing, grunting | Examples: Echolalia (repeating words or phrases spoken by others), palilalia (rapid repetition of one’s own words or phrases), and coprolalia (compulsive utterance of obscene words or phrases) | |
| Coprolalia is not pathognomonic of tourette syndrome. In fact less than 10% of tourette syndrome patients exhibit coprolalia. Hence, coprolalia is not required in diagnosing tourette syndrome | ||
| Motor | Simple motor tics: Rapid, meaningless contractions of one or a few muscles | Complex motor tics: Less common, typically more purposeful movements with a slower and longer nature. The movements appear more coordinated and may involve a cluster of movements |
| Examples: Eye blinking, shoulder shrugging, head jerking, hand clapping, neck stretching, mouth movements, head, arm or leg jerks, and facial grimacing | Examples: Facial gestures, dystonic postures, throwing, arm thrusting, touching objects or people, stereotyped imitation of the movements (echopraxia) and obscene gestures (copropraxia) |
Although the American Psychiatric Association Diagnostic and Statistical Manual, Fifth Edition (DSM-5) diagnostic criteria for TS necessitate the presence of both multiple motor and one or more vocal tics, they need not occur concurrently. The tics may wax and wane in frequency and must have lasted for more than one year since first onset. Substance induced etiologies, for example cocaine use, and general medical conditions such as Huntington’s disease or post viral encephalitis, are exclusionary criteria[1].
The Tourette Syndrome Classification Study Group defined TS as the presence of motor and vocal tics with frequent tics almost daily for at least one year, with an onset before age 21. In addition, the symptoms should be observed by an examiner[3]. See Table 2 for definitions and classification of tic disorders.
Table 2.
Definitions and classification of tic disorders
| Classification of tic syndromes/tourette's disorder. Tourette Syndrome Classification Study Group. Tourette Syndrome Criteria: (TSCSG 1993) |
| A Both multiple motor and one or more vocal tics have been present at some time during the illness, although not necessarily concurrently |
| B The tics occur many times a day, nearly every day, or intermittently throughout a period of more than a year |
| C The anatomic location, number, frequency, complexity, type, severity of tics changes over time |
| D Onset before age 21 |
| E Involuntary movements and noises cannot be explained by other medical conditions |
| F Motor and/or vocal tics must be witnessed by a reliable examiner directly at some point in the illness or be recorded by videotape or cinematography |
The prevalence of tic disorders in the classroom is between 5% and 20% of children, with impairments occurring in 1-10 per 1000[4-6]. Tic disorders have comorbidity with other disorders including obsessive compulsive disorder (OCD), attention deficit hyperactivity disorder (ADHD), learning difficulties and sleep abnormalities[2,5,6]. Leckman[7] (2002), reported the average age of onset of TS symptoms as seven years with a range from three to eight years. However, the DSM-5 gives the average tic onset age as between four and six years with peak severity being between 10 and 12 years[1]. See Figure 1 for clinical course of TS.
Figure 1.
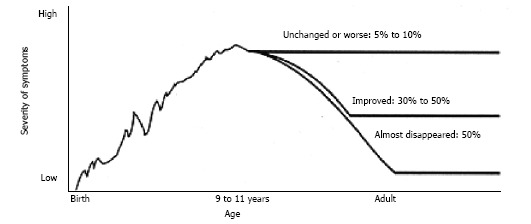
Onset of Tourette syndrome is before 7 years of age. It is usually recognized 2-3 years after onset. TS peak severity is at 9-11 years of age. Approximately 5%-10% have intensifying course. Approximately 85% experience a reduction of symptoms during and after adolescence[9]. TS: Tourette syndrome.
ADHD is the most common childhood psychiatric disorder with an estimated prevalence of 2% to 15%[8]. The onset of ADHD symptoms is usually between four and five years of age, thus generally appearing before the onset of tics[9]. ADHD related symptoms have been reported in 35% to 90% of children with TS[2,4,5,10]. TS with concomitant ADHD can cause significant psychosocial and behavioral impairments[11]. We present two cases of adolescent TS associated with ADHD and hypothesize that stimulant medications did not exacerbate the former disorder in these patients.
CASE REPORT
Case 1
An 8-year-old boy was referred to the child psychiatric clinic by his school due to his use of inappropriate language. He was diagnosed with ADHD at 4 years of age. At age six he was treated with stimulants, including methylphenidate and mixed amphetamine salts. Significant improvement of his ADHD symptoms occurred with immediate release mixed amphetamine salts, but the medication was discontinued due to excessive blinking and bilateral hand tics. Of note, the child’s maternal uncle had a history of TS. The stimulant was discontinued and, 4 wk later, the tics subsided. However the blinking continued intermittently. As a result, child neurology was consulted and clonidine 0.1 mg at night was prescribed to manage the ADHD and insomnia. On this regimen the boy’s parents noted a lessening of his hyperactivity and impulsivity, but a continued poor attention span. The child started using profanity at school, which led to his being bullied as well as his eventual suspension from school. Both the neurologist and the child psychiatrist agreed to re-evaluate the child for possible TS. The neurological examination was essentially normal. His EKG and routine blood tests, including a CBC, CMP and a lead level, were non-significant. Based on his history and clinical examination, a diagnosis of TS was made. He was started on immediate-release dexmethylphenidate 5 mg twice daily, gradually increased after 4 wk to 10 mg twice daily for the ADHD related symptoms. His regimen was later simplified by changing to a morning dose of 25 mg of the extended-release form. The core TS symptoms were treated with risperidone 0.125 mg twice daily, increased after 6 wk to a maintenance dose of 0.5 mg twice daily. The severity of his tics was monitored using the Yale Global Tic Severity Scale (YGTSS). After 6 mo of treatment, the boy’s vocal tics completely subsided. He continued to have intermittent blinking, but with a significant reduction in frequency from once every few minutes to less than 10 times per day.
Case 2
A 10-year-old boy with a 4 year history of ADHD combined type, as well as a learning disorder, experienced an initial good response to extended release methylphenidate. However, after 8 mo of management with this formulation at 36 mg each morning, his parents discontinued the medication due to worsening bilateral eye blinking. The methylphenidate was replaced with 10 mg of atomoxetine daily, with a gradual increase over a 2 mo period to 100 mg daily. After 2 mo on this regimen, the boy’s ADHD symptoms worsened, the frequency of blinking increased and he experienced vocal tics. During re-evaluation, the child reported a history of “cursing” people for more than 2 years. He could avoid directing profanity at others by retreating to the restroom to curse. After developing a good alliance with his psychiatrist, he reported having a secret “dictionary,” which was coprographic in nature, listed profane words beginning with A through Z, and included obscene drawings. The boy regularly read the dictionary to decrease the urge to directly curse at others. After a comprehensive evaluation, he was diagnosed with TS and comorbid ADHD. The ADHD symptoms were well controlled on 15 mg of immediate-release dexmethylphenidate in the morning and 10 mg in the afternoon. Clonidine 0.05 mg at night was added to treat his TS symptoms. He experienced drowsiness which subsided after 3 wk and within 2 mo clonidine was increased to 0.1 mg twice daily. The boy’s ADHD and TS symptoms significantly subsided on the combination of dexmethylphenidate and clonidine.
DISCUSSION
TS affects people of all racial and ethnic groups. The Centers for Disease Control and Prevention (CDC) reports that the disease is more likely among non-Hispanic white people than among Hispanic or African American individuals[12]. There is also a predilection in males three times that of females. In the United States, an estimated 3 in 1000 children between 6 and 17 years of age have TS, with an incidence twice as high among 12-17 year olds vs 6-11 year olds[12]. The prevalence of the disorder varies between 0.4% and 3.8% internationally, with a lower rate in sub-Saharan Black Africans[6,13-15]. In the United Kingdom the prevalence is between 0.46% and 1.85% for those between the ages of 5 and 18, with an average prevalence of 1%[5,13].
Multifactorial pathogeneses have been attributed to TS. There is a prevalence of 5% to 15% in first-degree relatives of those with TS. A higher ratio of concordance in monozygotic twins as compared to dizygotic twin pairs exists[5,6]. The mode of inheritance is believed to operate mainly through a dominant gene. Boys with the gene(s) are three or more times likely than girls to manifest TS symptoms[12]. Longitudinal studies show some evidence that gender and stress-related hormonal factors are entwined in the disorder’s pathogenesis[16]. There is also speculation about the role of gonadal androgens during the very early stages of central nervous system development in utero. Some clinical trials support the view that a change in the hormonal contexture during adolescence and adulthood affects tic severity[17]. In addition, monoamine neurotransmission has been implicated in the neurobiology of TS. Positron emission tomography and single-photon emission computer tomography studies suggest an abnormal regulation of dopamine production and metabolism in TS leading to higher dopamine levels[5], and lower levels of serotonin and glutamate have been found in such individuals[5]. Brain findings are usually normal in these patients. However, in a subpopulation, brain magnetic resonance imaging (MRI) scan studies have demonstrated an increased number of subcortical hyperintensities and reduced neuronal activity in the basal ganglia. Increased brain activity in the prefrontal, parietal, temporal, and cingulate regions has also been reported[2,5,6]. Volumetric imaging studies have demonstrated smaller caudate volumes[2,5,6,9,14,15]. Furthermore, children with TS tend to have smaller corpus callosum, while adults with TS have larger corpus callosums as compared to the normal population[2,5,6,9,14,15]. The TS subgroups with ADHD comorbidity appear to have increased left amygdala volume as compared to those without comorbidity[18,19]. Other implicated etiological factors of TS include intrauterine exposure to alcohol and cigarette smoke, a complicated birth, and low birth weight[2,14,15]. Possible autoimmune causes have also been considered based on studies linking TS and exposure to group A β-hemolytic streptococcal infections (GABHS) complicated by Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infections (PANDAS)[7,12].
A TS diagnosis is based on clinical history and examination. When clinically indicated, routine laboratory and radiological investigations should be considered to rule out other causes of tic disorders. See Table 3 for common differential diagnoses of TS[1,3,6,9,12,13,20]. In order to document the quality and quantity of tics, some experts recommend video-recording by parents and teachers[2]. A standardized rating scale such as the YGTSS may be useful for diagnosing and monitoring treatment response[5,21]. Although brain MRI scans will likely be normal in TS, brain imaging is indicated in those suspected of having neuroinflammatory/degenerative conditions, for example Sydenham’s chorea. In addition, DNA testing should be considered in individuals with family history of Huntington’s chorea (especially DNA microarray technology)[2,5,6]. Heavy metal toxicities should be considered, including lead, as well as serum copper and ceruloplasmin if Wilson’s disease is suspected[5,6,14,15]. Electroencephalography (EEG) may be useful when myoclonic epilepsy is suspected[2,14,15]. A throat swab should be considered in patients who have history of pharyngitis to rule out GABHS and PANDAS[2,5,6,9,12,14,15].
Table 3.
Common differential diagnoses of tics
| Stroke |
| Dystonia |
| PANDAS |
| Encephalitis |
| Head trauma |
| Epileptic seizures |
| Sydenham’s chorea |
| Carbon monoxide poisoning |
| Functional movement disorders in children |
| Chromosomal disorders such as Down syndrome and Fragile X syndrome |
| Genetic conditions (such as Huntington’s disease, Wilson’s disease and Tuberous sclerosis) |
| Stereotypy (in developmental disorders such as Autism spectrum disorders and Stereotypic movement disorder) |
| Medication-induced tics (i.e., Neuroleptics, Stimulants, Antiepileptics, Lithium) |
PANDAS: Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infections.
The CDC has conducted a number of studies examining comorbidities in TS. Children and adolescents with TS have higher risk of comorbid learning, behavioral, and social problems. Approximately, 79% of children with TS have at least one comorbid mental health, behavioral or developmental condition[12,22]. Among children with TS, 64% have ADHD, 43% have behavioral problems such as oppositional defiant disorder or conduct disorder, 40% have anxiety, 36% suffer from depression, and 28% are developmentally delayed[22]. See Figure 2 for prevalence of comorbid diagnoses among 6 to 17 year olds with TS[22]. Other clinical symptoms associated with TS include stuttering, aggressive and antisocial behavior, impulsivity, exhibitionism, sleep disturbances and self-injurious behaviors[5]. Since ADHD is the highest comorbid condition with TS, it is essential for clinicians to be familiar with the diagnosis and management of these conditions.
Figure 2.
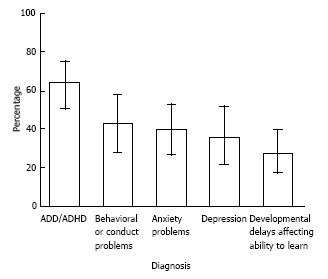
Adapted with permission from CDC. Prevalence of selected diagnoses among persons aged 6-17 years with tourette syndrome. ADD: Attention deficit disorder; ADHD: Attention deficit hyperactivity disorder.
In recent years, there has been considerable research on the psychopharmacology of TS associated with ADHD. The psychopharmacological management of children with TS associated with ADHD should be tailored towards the clinical presentation and severity of the illness[11,23]. Leckman et al[20] 2002, suggested prioritization of pharmacological interventions based on the degree of distress and impairment. Since the natural course of tics is either short-term intermittent episodes or long-term waxing and waning of symptoms, medication options should be tailored to minimize side effects while maximizing treatment benefits[2,20]. Pharmacological management of TS associated with ADHD is similar to other childhood psychiatric disorders in that few of the medications commonly utilized are FDA approved[24].
Several studies have demonstrated the effectiveness of stimulants in alleviating ADHD symptoms. Contrary to previous notions that stimulants can worsen tics in TS, some studies have shown contrary results. A critical review of the literature reported that group data analysis showed no significant increase in tics when stimulants are used in patients with tics as compared with controls[10]. This conclusion was also supported by the Tourette Syndrome Study Group in a multicenter, randomized, double-blind 16 wk clinical trial in which 136 children with ADHD and a chronic tic disorder were randomly administered clonidine, methylphenidate, combined clonidine and methylphenidate, or placebo. The group concluded that the combination of methylphenidate and clonidine is effective for ADHD in children with comorbid tics and that prior recommendations to avoid methylphenidate because of concerns of worsening tics are unsupported by the trial[25].
Studies have shown that the motor and behavioral symptoms associated with TS respond well to most typical and atypical antipsychotic medications. The typical antipsychotic drugs, such as haloperidol, fluphenazine and pimozide, with a high tendency to block postsynaptic dopamine (D2 receptors) are the treatment of choice due to their greater effectiveness[2,22,26]. However, typical antipsychotics remain a second-line treatment option because of side effects including extrapyramidal side-effects (EPSE) and tardive dyskinesia (TD) for haloperidol, and cardiotoxicity for pimozide[22,26]. Other side-effects include sedation, orthostatic hypotension, dry eyes and mouth, urinary retention and confusion. Neuroleptic malignant syndrome (NMS) is a rare but serious adverse-event characterized by lead pipe rigidity, autonomic instability, increased heart rate, fever, and rhabdomyolysis.
The atypical neuroleptic medications such as risperidone, ziprasidone, olanzapine, aripiprazole, and quetiapine, which selectively block postsynaptic dopamine D2 receptors, have had some encouraging outcomes in the treatment of tics associated with TS[2,22,23,26,27]. Close monitoring when using these medications is essential especially in pediatric populations with higher risks of developing metabolic syndrome and EPSE. The mechanisms of action of atypical neuroleptic drugs are different from conventional medications in that atypicals have greater affinity for serotonin receptors (especially 5-HT2A) than D2 receptors, thus generally causing minimal EPSE, milder increases in prolactin and lesser tendencies to induce TD[28,29]. Risperidone is associated with orthostasis, and more EPSE than other atypical agents. It also causes significant hyperprolactinemia which in turn can cause adverse events including gynecomastia in boys. Prolactin monitoring has been recommended in those treated with risperidone. Olanzapine is associated with higher incidences of sedation and metabolic syndrome. Quetiapine can lead to sedation and anti-cholinergic side effects, while ziprasidone can cause dry ejaculation and a prolonged Q-T interval[2,22,23,26-29].
Clonidine and guanfacine are sympatholytic agents that lower blood pressure and heart rate. Their mechanisms of action involve alpha-2A adrenoceptor selective agonists. The use of this group of medications for TS is supported by a few controlled studies[22,26]. Clonidine in divided daily doses, ranging from 0.1 to 0.3 mg, has been associated with favorable outcomes in pediatric populations[2,22,23,26,30]. Also, guanfacine in divided daily doses of 0.5 to 3 mg has been recommended for milder TS[2,22,23,26]. Associated adverse-effects of clonidine and guanfacine include sedation, dry mouth, headache, postural hypotension and dizziness, and sudden discontinuation can induce a hypertensive crisis. It is essential to monitor blood pressure and heart rate and obtain a baseline EKG when using clonidine or guanfacine[23,31,32]. Sudden discontinuation, especially of clonidine, should be avoided due to risk of rebound hypertension.
The data are mixed concerning the use of antiepileptic drugs such as levetiracetam and topiramate in the treatment of TS[22,33]. Other reported alternative pharmacological treatments of tics in TS include tetrabenazine, ropinirole, botulinum toxin, baclofen and clonazepam but the evidence is limited[2,22,23,26,34].
Since this paper focuses on the psychopharmacological management of TS and ADHD, psychological interventions are not addressed in detail. There are evidence-based non-pharmacological treatment options for TS associated with ADHD[35,36]. With regard to non-psychopharmacological management of tics, studies have revealed better outcomes from habit reversal therapy and, exposure and response prevention strategies[35,36]. In addition, treating OCD symptoms associated with TS may potentially reduce tics and ADHD symptoms[26,36,37]. Selective serotonin re-uptake inhibitor (SSRI) antidepressants are the preferred pharmacological treatment for OCD. Multiple studies support the role of cognitive behavioral therapy (CBT) for behavioral management of OCD in TS[26,36-38].
Recent studies have revealed promising outcomes from transcranial magnetic stimulation (TMS)[39,40] and deep brain stimulation surgery[41-43] in the treatment of medication resistant tics associated with TS.
In conclusion, ADHD has a high correlation with TS and patients with ADHD are more likely to develop tic disorders, with or without treatment with stimulants. Tics which appear during the treatment of ADHD with stimulants may be due to a naturally developing tic disorder in which the tics have the usual waxing and waning pattern of occurrence, intensity, and frequency and may have developed even without the use of stimulants. The decision to use, or to continue to use, stimulants must be made on a case-to-case basis. Overall the treatment of ADHD with appropriate psychopharmacological agents, including stimulants, is suggested if the treatment benefits outweigh the potential medication risks.
Medication management of TS associated with ADHD is quite variable. The choice of appropriate pharmacological agent(s) should depend on the severity of the impairments. Starting with a non-stimulant agent such as guanfacine or clonidine is recommended, as they are effective in alleviating both symptoms of TS and ADHD. The newer long acting preparations of guanfacine approved for ADHD may be the most reasonable agents to consider first. If the TS is not severe, but the ADHD is disabling, the option of treating the ADHD with a stimulant should be considered. The course and severity of tics should be monitored with a reliable TS rating scale, such as the YGTSS. It is also recommended that, in order to prevent any future debilitating disease process, careful history taking be done in diagnosing and managing of symptoms of TS associated with ADHD.
ACKNOWLEDGMENTS
The authors wish to thank the following individuals for their useful feedback with regard to the case reports: Mark Reber MD, William Sonis MD, and Karim Ghobrial-Sedky, MD.
COMMENTS
Case characteristics
Two boys, ages 8 and 10 years old, with Attention deficit hyperactivity disorder (ADHD) are treated with stimulants and experience the emergence of tics, later diagnosed as tourette syndrome (TS).
Clinical diagnosis
Case 1: Inappropriate language/profanity, vocal tics and inattention, impulsivity and hyperactivity. Case 2: Bilateral blinking, vocal tics, profanity, impulsivity and hyperactivity.
Differential diagnoses
With regard to the etiology of tics, Stroke, Dystonia, Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infections, Encephalitis, Head trauma, Epileptic seizures, Sydenham’s chorea, Carbon monoxide poisoning, Functional movement disorders, Chromosomal and Genetic disorders, Stereotypy and Medication-induced tics should be considered.
Laboratory diagnosis
All laboratory studies were within normal limits.
Pathological diagnoses
Both cases were examples of TS associated with ADHD.
Treatment
In both cases stimulants were used to treat ADHD, however the TS was treated with clonidine in the first case and risperidone in the second.
Related reports
Tics can present as an adverse effect of stimulants. However, given the high comorbidity of ADHD with TS, emerging tics can be symptom of TS, which typically manifests temporally later than ADHD. In the cases presented, both boys were changed to non-stimulant medications after developing tics, which led to suboptimal treatment of their ADHD symptoms. The second child actually experienced a worsening of TS symptoms on the non-stimulant medication. Despite providers’ reluctance to prescribe stimulants due to fear of causing tics or worsening pre-existing tics, evidence has shown that stimulants can be beneficial in the treatment of TS associated with ADHD. In fact, using stimulants may reduce the severity of the tics and improve the quality of life of patients with these disorders.
Term explanation
TS is a neurodevelopmental disorder characterized by multiple chronic motor and vocal tics developing before adulthood. ADHD, also a neurodevelopmental disorder, presents with a persistent or on-going pattern of inattention and/or hyperactivity and impulsivity, which interferes with typical development and quality of life. ADHD is associated with difficulties in achieving and sustaining attention, and with executive function and working memory.
Experiences and lessons
There is a misperception that stimulants are contraindicated in patients with tic disorders due to the potential to cause or exacerbate tics, however, the role of stimulants in the treatment of TS, when the benefits outweigh the risks, cannot be over emphasized.
Peer-review
The manuscript is an informative review regarding the diagnosis and management of Tourette syndrome associated with ADHD. It is deemed to be of academic value and worthy of publishing.
Footnotes
Institutional review board statement: This case report was exempted by the Institutional Review Board standards at the Drexel University College of Medicine, Philadelphia, PA, United States.
Informed consent statement: The legal guardians of the patients involved in this study provided informed written consent authorizing the use and disclosure of the respective patients protected health information. The identities of the patients were protected by using non-identifiable biographic data.
Conflict-of-interest statement: The authors have no financial disclosures and no relationship with any pharmaceutical company whose products are mentioned in this case report or with the manufacturers of competing products.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 2, 2015
First decision: November 6, 2015
Article in press: January 7, 2016
P- Reviewer: Classen CF, Sangkhathat S S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders fifth edition. Washington (DC): American Psychiatric Association; 2013. [Google Scholar]
- 2.Cohen DJ, Jankovic J, Goetz CG. Advances in neurology V. 85 Tourette syndrome. Philadelphia: Lippincott Williams Wilkins; 2001. [PubMed] [Google Scholar]
- 3.The Tourette Syndrome Classification Study Group. Definitions and classification of tic disorders. Arch Neurol. 1993;50:1013–1016. doi: 10.1001/archneur.1993.00540100012008. [DOI] [PubMed] [Google Scholar]
- 4.Snider LA, Seligman LD, Ketchen BR, Levitt SJ, Bates LR, Garvey MA, Swedo SE. Tics and problem behaviors in schoolchildren: prevalence, characterization, and associations. Pediatrics. 2002;110:331–336. doi: 10.1542/peds.110.2.331. [DOI] [PubMed] [Google Scholar]
- 5.Tallur K, Minns RA. Tourette’s syndrome. Paediatr Child Health. 2010;20:88–93. [Google Scholar]
- 6.Robertson Jr WC, Talavera F, Kao , A , Sheth RD. Tourette syndrome and Other Tic Disorders. Emedicine/Medscape. Available from: http://emedicine.medscape.com/article/1182258-overview.
- 7.Leckman JF. Tourette’s syndrome. Lancet. 2002;360:1577–1586. doi: 10.1016/S0140-6736(02)11526-1. [DOI] [PubMed] [Google Scholar]
- 8.Robertson MM. Tourette syndrome, associated conditions and the complexities of treatment. Brain. 2000;123 Pt 3:425–462. doi: 10.1093/brain/123.3.425. [DOI] [PubMed] [Google Scholar]
- 9.Bagheri MM, Kerbeshian J, Burd L. Recognition and management of Tourette’s syndrome and tic disorders. Am Fam Physician. 1999;59:2263–272, 2274. [PubMed] [Google Scholar]
- 10.Erenberg G. The relationship between tourette syndrome, attention deficit hyperactivity disorder, and stimulant medication: a critical review. Semin Pediatr Neurol. 2005;12:217–221. doi: 10.1016/j.spen.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Singer HS. Treatment of tics and tourette syndrome. Curr Treat Options Neurol. 2010;12:539–561. doi: 10.1007/s11940-010-0095-4. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Tourette Syndrome (TS) Available from: http://www.cdc.gov/ncbddd/tourette/index.html.
- 13.Robertson MM. Diagnosing Tourette syndrome: is it a common disorder? J Psychosom Res. 2003;55:3–6. doi: 10.1016/s0022-3999(02)00580-9. [DOI] [PubMed] [Google Scholar]
- 14.Robertson MM. The prevalence and epidemiology of Gilles de la Tourette syndrome. Part 1: the epidemiological and prevalence studies. J Psychosom Res. 2008;65:461–472. doi: 10.1016/j.jpsychores.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Robertson MM. The prevalence and epidemiology of Gilles de la Tourette syndrome. Part 2: tentative explanations for differing prevalence figures in GTS, including the possible effects of psychopathology, aetiology, cultural differences, and differing phenotypes. J Psychosom Res. 2008;65:473–486. doi: 10.1016/j.jpsychores.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Chappell P, Riddle M, Anderson G, Scahill L, Hardin M, Walker D, Cohen D, Leckman J. Enhanced stress responsivity of Tourette syndrome patients undergoing lumbar puncture. Biol Psychiatry. 1994;36:35–43. doi: 10.1016/0006-3223(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 17.Leckman JF, Peterson BS. The pathogenesis of Tourette’s syndrome: epigenetic factors active in early CNS development. Biol Psychiatry. 1993;34:425–427. doi: 10.1016/0006-3223(93)90232-3. [DOI] [PubMed] [Google Scholar]
- 18.Peterson BS, Choi HA, Hao X, Amat JA, Zhu H, Whiteman R, Liu J, Xu D, Bansal R. Morphologic features of the amygdala and hippocampus in children and adults with Tourette syndrome. Arch Gen Psychiatry. 2007;64:1281–1291. doi: 10.1001/archpsyc.64.11.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wittfoth M, Bornmann S, Peschel T, Grosskreutz J, Glahn A, Buddensiek N, Becker H, Dengler R, Müller-Vahl KR. Lateral frontal cortex volume reduction in Tourette syndrome revealed by VBM. BMC Neurosci. 2012;13:17. doi: 10.1186/1471-2202-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, Cohen DJ. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989;28:566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC) Prevalence of diagnosed Tourette syndrome in persons aged 6-17 years - United States, 2007. MMWR Morb Mortal Wkly Rep. 2009;58:581–585. [PubMed] [Google Scholar]
- 22.Eddy CM, Rickards HE, Cavanna AE. Treatment strategies for tics in Tourette syndrome. Ther Adv Neurol Disord. 2011;4:25–45. doi: 10.1177/1756285610390261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King AR, Scahill L, Lombroso JP, Leckman J. Tourette’s syndrome and Other Tic Disorders. In: Martin A, Scahill L, Charney SD, Leckman FJ, editors. Pediatric Psychopharmacology: Principles and Practice. Oxford: Oxford University Press; 2002. pp. 526–542. [Google Scholar]
- 24.Sood R, Coffey BJ. Pharmacotherapeutic challenges in the management of attention-deficit/hyperactivity disorder and chronic tics in a school aged child. J Child Adolesc Psychopharmacol. 2013;23:628–631. doi: 10.1089/cap.2013.2392. [DOI] [PubMed] [Google Scholar]
- 25.Tourette’s Syndrome Study Group. Treatment of ADHD in children with tics: a randomized controlled trial. Neurology. 2002;58:527–536. doi: 10.1212/wnl.58.4.527. [DOI] [PubMed] [Google Scholar]
- 26.Shavitt RG, Hounie AG, Rosário Campos MC, Miguel EC. Tourette’s Syndrome. Psychiatr Clin North Am. 2006;29:471–486. doi: 10.1016/j.psc.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Waldon K, Hill J, Termine C, Balottin U, Cavanna AE. Trials of pharmacological interventions for Tourette syndrome: a systematic review. Behav Neurol. 2013;26:265–273. doi: 10.3233/BEN-2012-120269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srour M, Lespérance P, Richer F, Chouinard S. Psychopharmacology of tic disorders. J Can Acad Child Adolesc Psychiatry. 2008;17:150–159. [PMC free article] [PubMed] [Google Scholar]
- 29.Budman CL. The role of atypical antipsychotics for treatment of Tourette’s syndrome: an overview. Drugs. 2014;74:1177–1193. doi: 10.1007/s40265-014-0254-0. [DOI] [PubMed] [Google Scholar]
- 30.Scahill L, Erenberg G, Berlin CM, Budman C, Coffey BJ, Jankovic J, Kiessling L, King RA, Kurlan R, Lang A, et al. Contemporary assessment and pharmacotherapy of Tourette syndrome. NeuroRx. 2006;3:192–206. doi: 10.1016/j.nurx.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newcorn HJ, Clerkin S, Schulz PK, Halperin MJ. Alpha adrenergic agonists: Clonidine and guanfacine. In: Andrés Martin, Lawrence Scahill, Christopher Kratochvil., editors. Pediatric Psychopharmacology: Principles and Practice. 2nd ed. Oxford: Oxford University Press; 2011. pp. 263–274. [Google Scholar]
- 32.Roessner V, Schoenefeld K, Buse J, Bender S, Ehrlich S, Münchau A. Pharmacological treatment of tic disorders and Tourette Syndrome. Neuropharmacology. 2013;68:143–149. doi: 10.1016/j.neuropharm.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 33.Jankovic J, Jimenez-Shahed J, Brown LW. A randomised, double-blind, placebo-controlled study of topiramate in the treatment of Tourette syndrome. J Neurol Neurosurg Psychiatry. 2010;81:70–73. doi: 10.1136/jnnp.2009.185348. [DOI] [PubMed] [Google Scholar]
- 34.Singer HS, Wendlandt J, Krieger M, Giuliano J. Baclofen treatment in Tourette syndrome: a double-blind, placebo-controlled, crossover trial. Neurology. 2001;56:599–604. doi: 10.1212/wnl.56.5.599. [DOI] [PubMed] [Google Scholar]
- 35.Cavanna AE, Seri S. Tourette’s syndrome. BMJ. 2013;347:f4964. doi: 10.1136/bmj.f4964. [DOI] [PubMed] [Google Scholar]
- 36.Shprecher D, Kurlan R. The management of tics. Mov Disord. 2009;24:15–24. doi: 10.1002/mds.22378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piacentini J, Woods DW, Scahill L, Wilhelm S, Peterson AL, Chang S, Ginsburg GS, Deckersbach T, Dziura J, Levi-Pearl S, et al. Behavior therapy for children with Tourette disorder: a randomized controlled trial. JAMA. 2010;303:1929–1937. doi: 10.1001/jama.2010.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilhelm S, Peterson AL, Piacentini J, Woods DW, Deckersbach T, Sukhodolsky DG, Chang S, Liu H, Dziura J, Walkup JT, et al. Randomized trial of behavior therapy for adults with Tourette syndrome. Arch Gen Psychiatry. 2012;69:795–803. doi: 10.1001/archgenpsychiatry.2011.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mantovani A, Lisanby SH, Pieraccini F, Ulivelli M, Castrogiovanni P, Rossi S. Repetitive transcranial magnetic stimulation (rTMS) in the treatment of obsessive-compulsive disorder (OCD) and Tourette’s syndrome (TS) Int J Neuropsychopharmacol. 2006;9:95–100. doi: 10.1017/S1461145705005729. [DOI] [PubMed] [Google Scholar]
- 40.Mantovani A, Leckman JF, Grantz H, King RA, Sporn AL, Lisanby SH. Repetitive Transcranial Magnetic Stimulation of the Supplementary Motor Area in the treatment of Tourette Syndrome: report of two cases. Clin Neurophysiol. 2007;118:2314–2315. doi: 10.1016/j.clinph.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 41.Servello D, Porta M, Sassi M, Brambilla A, Robertson MM. Deep brain stimulation in 18 patients with severe Gilles de la Tourette syndrome refractory to treatment: the surgery and stimulation. J Neurol Neurosurg Psychiatry. 2008;79:136–142. doi: 10.1136/jnnp.2006.104067. [DOI] [PubMed] [Google Scholar]
- 42.Bajwa RJ, de Lotbinière AJ, King RA, Jabbari B, Quatrano S, Kunze K, Scahill L, Leckman JF. Deep brain stimulation in Tourette’s syndrome. Mov Disord. 2007;22:1346–1350. doi: 10.1002/mds.21398. [DOI] [PubMed] [Google Scholar]
- 43.Dehning S, Mehrkens JH, Müller N, Bötzel K. Therapy-refractory Tourette syndrome: beneficial outcome with globus pallidus internus deep brain stimulation. Mov Disord. 2008;23:1300–1302. doi: 10.1002/mds.21930. [DOI] [PubMed] [Google Scholar]


