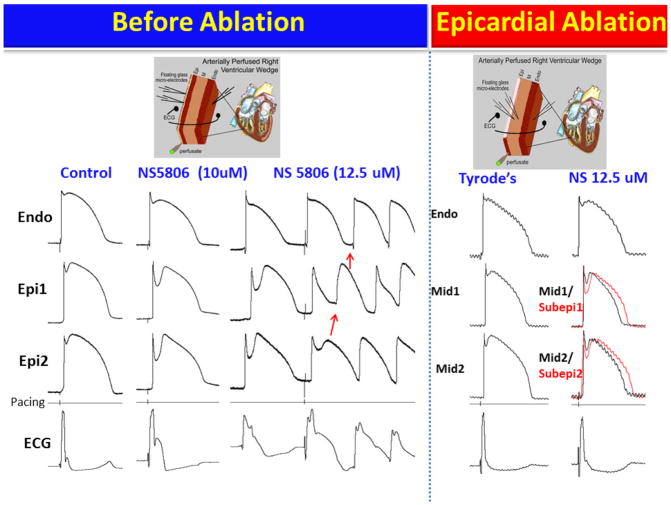
Figure 6.
Epicardial radiofrequency ablation abolishes the electrographic and arrhythmic manifestations of Brugada syndrome (BrS) in coronary-perfused canine right ventricular wedge-model. Transmembrane action potentials (AP) were simultaneously recorded from one endocardial (Endo) and two epicardial (Epi) sites together with a transmural pseudo-ECG. (The model is schematically illustrated in the top panels). Stimulus marker (pacing) is indicated in the 4th row. All recordings were obtained at 1000 ms basic cycle length.
Column 1: Control. Column 2: Recorded 20 min after the addition of the Ito-agonist NS5806 (10μM) to the coronary perfusate. The much greater accentuation of the Epicardial (Epi) vs. Endocardial (Endo) AP notch was associated with accentuation of the J wave in the ECG. Column 3: Recorded 15 min after increasing the concentration of NS5806 to 12.5 μM. A stimulated premature beat induced VT (later VF) via a phase 2 reentry (P2R) mechanism. The abbreviated cycle length caused loss of the AP dome in Epi1 due to use-dependent block of INa by NS5806. In the Endo and Epi2 sites the dome is maintained and the notch is slightly reduced because slow recovery of Ito overwhelmed the use-dependent inhibition of INa. The pronounced epicardial and transmural dispersion of repolarization created the substrate for P2R and VT/VF. Column 4: Recorded after washout of NS5806 and 70 min after RF ablation of the epicardial surface. APs were recorded from the subepicardial layer (Subepi) just below the ablation border and from the midmyocardium (Mid) because the Epi layer was destroyed. Column 5: Recorded 45 min after re-introduction of NS5806 (12.5μM) to the coronary perfusate. After ablation of epicardium, NS5806 was no longer able to induce the ECG or arrhythmic manifestations of BrS.