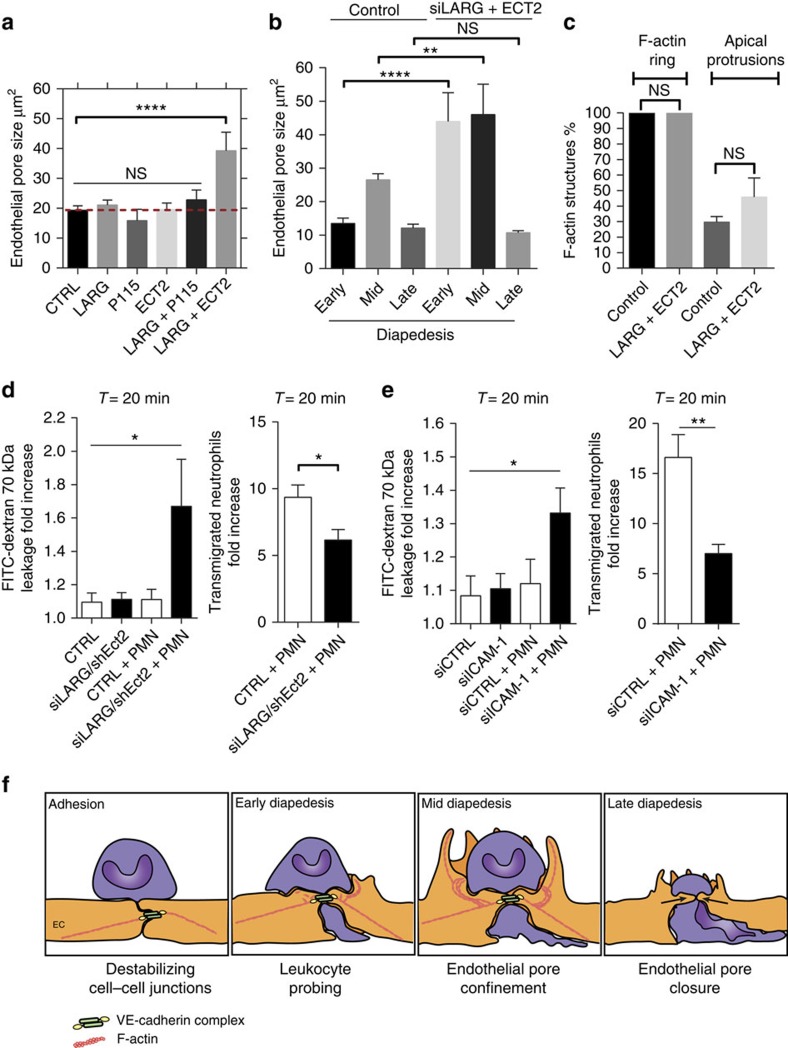
Figure 7. ICAM-1 regulates endothelial pore confinement through recruitment of the Rho GEFs LARG and Ect2.
(a) Quantification of endothelial pore size in LARG, p115RhoGEF or Ect2 depleted ECs. (b) Quantification of endothelial pore size during early, mid and late diapedesis in control versus LARG+Ect2 depleted ECs. (c) Quantification of F-actin-positive ring structures and F-actin-positive apical protrusions in control versus LARG+Ect2 depleted ECs.(d) Quantification of FITC–dextran and neutrophil extravasation after 20 min of neutrophil transmigration through control and Ect2/LARG (d) or ICAM-1-deficient ECs (e). (f) Model of endothelial pore formation. Adherent leukocytes destabilizing cell–cell junctions subsequently insert small pseudopodia between transient openings in the endothelium (leukocyte probing) during early diapedesis. The next step (mid diapedesis) involves local and transient RhoA activation that mediates endothelial pore confinement through the formation of a de novo basolateral F-actin ring and actomyosin contractility. Finally, persistent actomyosin contractility closes the endothelial pore behind transmigrating leukocytes. ICAM-1 is involved in the regulation of endothelial pore confinement through recruitment of the Rho GEFs LARG and Ect2. Basolateral F-actin ring formation and actomyosin contractility tightens the endothelial barrier during leukocyte diapedesis, making the leukocyte-induced endothelial pore impermeable for macromolecules. ****P<0.0001 (ANOVA) (a–c).**P<0.01 (ANOVA) (b) *P<0.05 (ANOVA) (e,d).*P<0.05 control versus siLARG/shEct2 (d) **P<0.01 control versus siICAM-1 (e) (Student's t-test). Data are representative of three independent experiments (a–e) with >12 transmigration events per group (a–c) (error bars (a–e) s.e.m). ANOVA, analysis of variance.