Figure 1. Metal dependence of PRORP2.
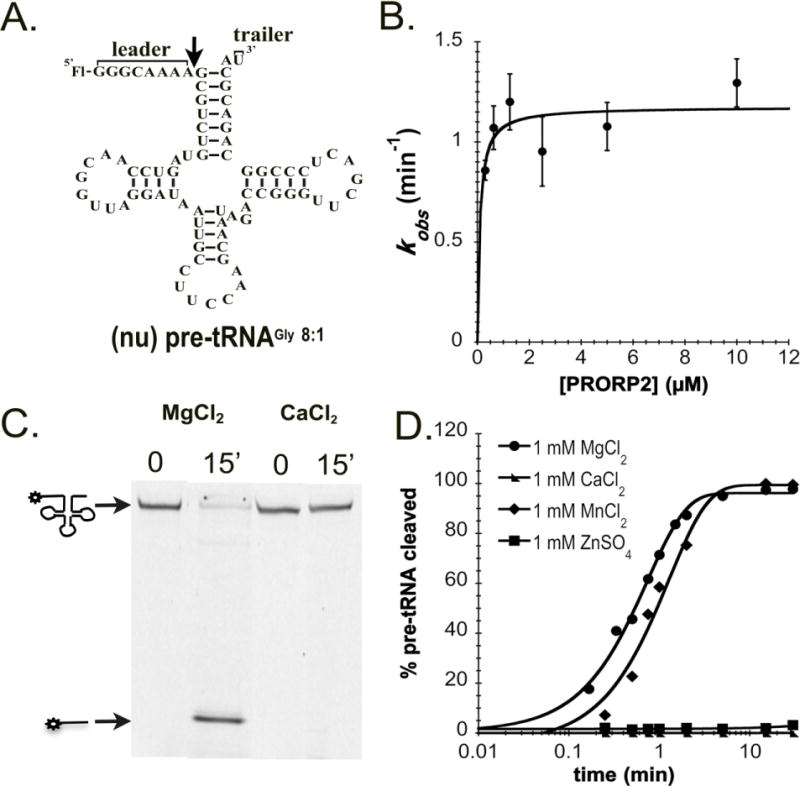
A. Proposed secondary structure of nuclear pre-tRNAGly 8:1 used in our in vitro assays. Pre-tRNAs are 5′ fluorescently labeled by fluorescein (Fl). The 5′ leader and 3′ trailer are noted, and the black arrow indicates the cleavage site. B. The dependence of the single turnover cleavage rate constant (kobs) on the PRORP2 concentration for (nu)pre-tRNAGly 8:1. C. Representative denaturing PAGE gel displaying the cleavage activity of PRORP2 for (nu)pre-tRNAGly 8:1 after a 15 min incubation with buffer containing 1 mM MgCl2 or CaCl2. Cleavage is observed in the presence of MgCl2 but not CaCl2 under standard reaction conditions. D. Representative timecourses for the cleavage of a 5′ “fluorescein-labeled” (nu)pre-tRNAGly 8:1 substrate catalyzed by PRORP2 under standard single turnover conditions in the presence of MgCl2 (circle), MnCl2 (diamond), ZnCl2 (square) and CaCl2 (triangle).
