Figure 4. Comparison of PRORP1 and PRORP2 crystal structures.
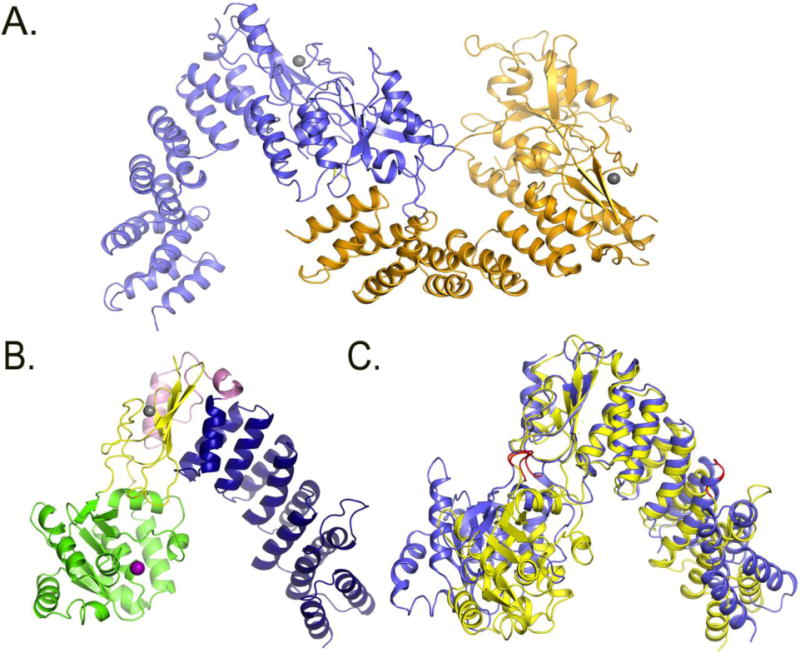
A. Crystal structure of the two monomers of PRORP2 in the asymmetric unit. B. Crystal structure of PRORP1. blue: PPR domain, yellow: central domain, Pink: A. thaliana specific insertion, green: metallonuclease domain, Grey: zink ion, Purple: modeled magnesium ion. C. PRORP1 and PRORP2 superimposition based on the central domains. The structure of PRORP2 (blue) is in an “open” conformation, while PRORP1 (yellow) is in a “closed” conformation. The predicted PRORP2 mechanical hinges are shown in red in the metallonuclease domain and the 3rd PPR motif.
