Figure 6. Comparison of the PPR, central and metallonuclease domains of PRORP1 and PRORP2.
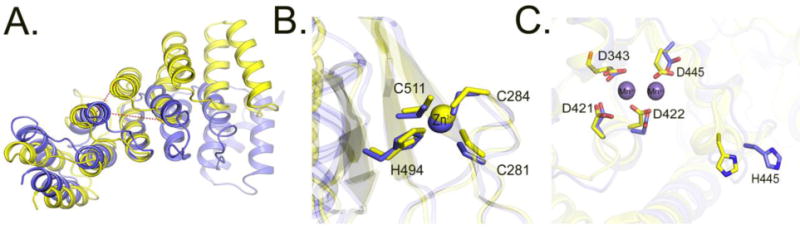
A. Superimposition of the 5th PPR motif of PRORP1 and PRORP2. Dotted red lines indicate differences in distance between the same residues in the 4th and 6th PPR helices in PRORP1 and PRORP2. B. Superimposition of the central domains of PRORP1 and PRORP2. Residues numbering correspond to the conserved amino acids in PRORP2. C. Superimposition of the active sites of PRORP1 (yellow) and PRORP2 (blue). Residue numbers correspond to conserved amino acids in PRORP2. Positions of Mn2+ ions are depicted based on the structure of PRORP1.
