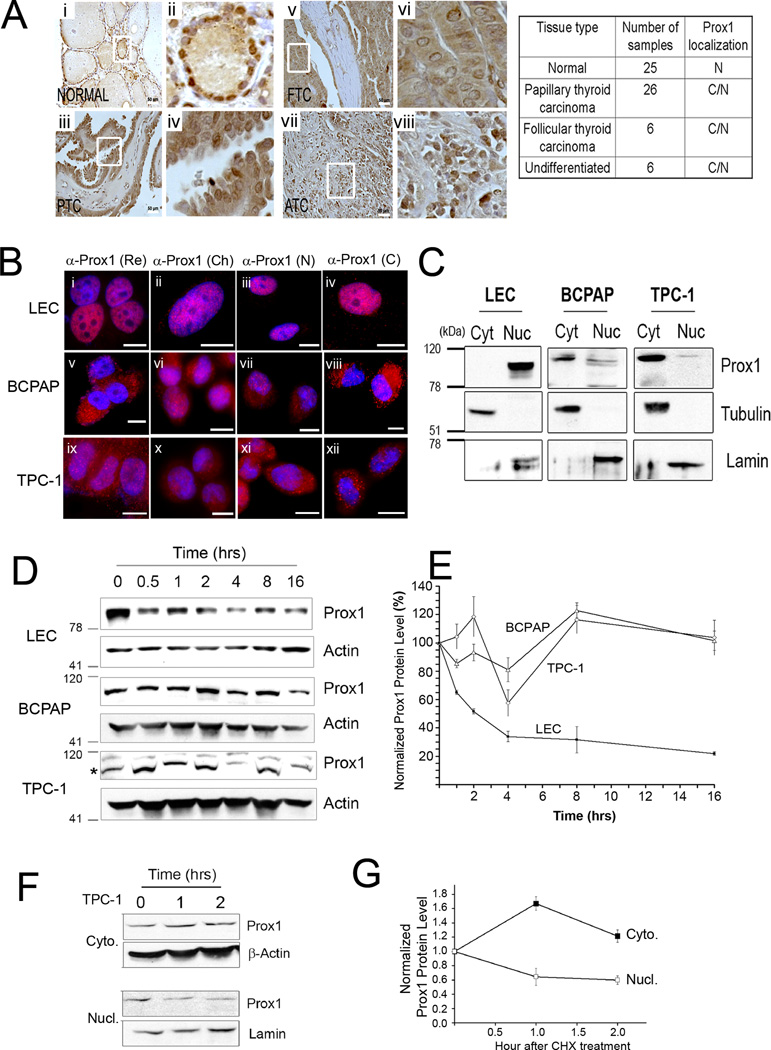
Figure 3. PROX1 protein is mislocalized to the cytoplasm and gains enhanced protein stability in thyroid cancer cells.
(A) IHC showing PROX1 localization in normal thyroid tissues (Normal, n=25), PTC (n=26), follicular thyroid carcinoma (FTC, n=6), anaplastic or undifferentiated thyroid carcinoma (ATC, n=6). Overview (i, iii, v, vii) are shown with enlarged images (ii, iv, vi, viii). Scale bars, 50 µm. PROX1 localization: nuclei (N), cytoplasm (C). (B) IF analyses showing PROX1 localization in LECs (i–iv), BCPAP (v–viii) and TPC1 (ix–xii). Four different PROX1 antibodies were used: rabbit polyclonal antibody from Reliatech (Re) (i,v,ix), mouse monoclonal antibody 5G10 from Millipore (Ch) (ii,vi,x), custom generated rabbit polyclonal antibodies against PROX1 N-terminus (N) (iii, vii,xi) and C-terminus (C) (iv,viii,xii). PROX1 was stained in red and the nuclei with DAPI. Scale Bars, 20 µm. (C) Western blot assays showing subcellular distribution of PROX1 protein. The cytoplasmic (cyt) and nuclear (nuc) fractions were obtained from LEC, BCPAP and TPC1, and probed for PROX1. Tubulin and lamin were also detected as the controls for the cytoplasmic and nuclear fractions, respectively. (D,E) PROX1 protein is more stable in PTC cells than in LECs. LECs, BCPAP and TPC1 cells were treated with cycloheximide (100 µg/ml) for the indicated time. Western blot analyses showing PROX1 protein (D). PROX1 band is marked with an asterisk for TPC-1 blot. PROX1 expression was normalized against β-actin (E). (F,G) Cytoplasmic PROX1 protein is more stable than nuclear PROX1. TPC1 cells were treated with cycloheximide (100 µg/ml) for 0, 1 and 2 hours, followed by subcellular fractionation. Western blotting for PROX1 in the cytoplasmic (Cyto.) and nuclear (Nucl.) fractions (F). β-actin and lamin were also detected as the controls for the cytoplasmic and nuclear fractions, respectively. Normalized band intensities of the cytoplasmic and nuclear PROX1 proteins to β-actin and lamin proteins, respectively (G). Error bars present standard deviations.