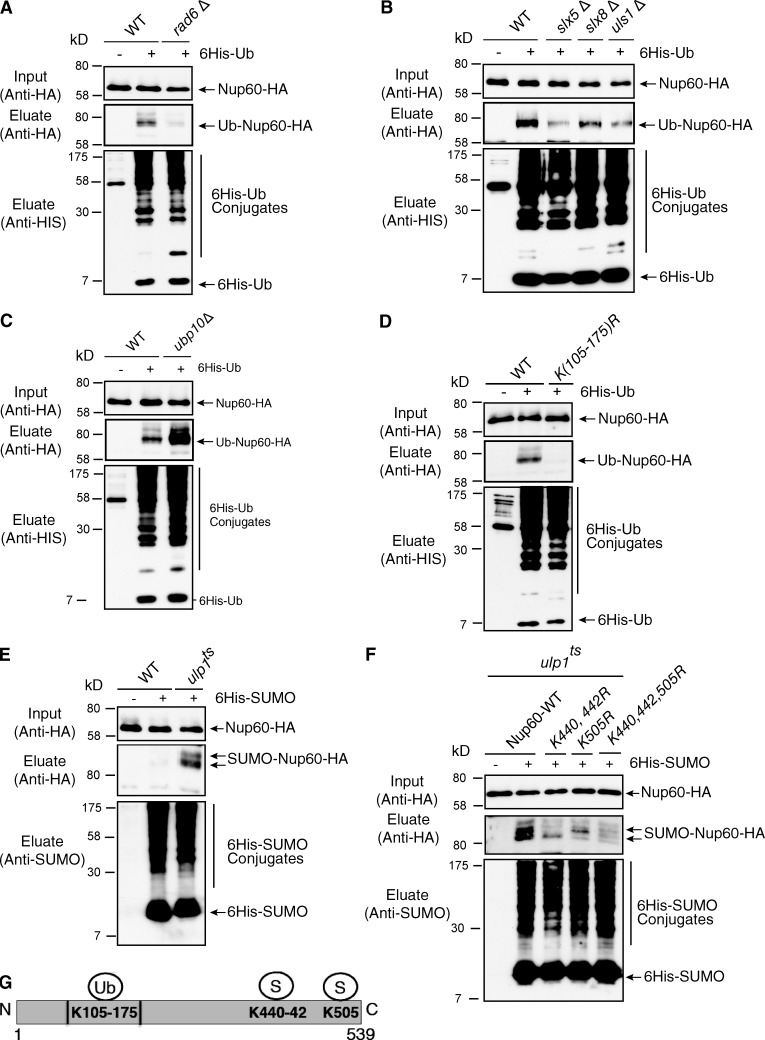
Figure 1.
Mechanisms of Nup60 ubiquitylation and SUMOylation. Ni-purified 6His-ubiquitin– (Ub) or 6His-SUMO–conjugated forms of Nup60-HA were extracted from cells transformed (+) or not transformed (−) with a plasmid encoding 6His-ubiquitin or 6His-SUMO, respectively, under control of the CUP1 promoter. Cell lysates (top) and Ni-purified material (middle) were examined by Western blotting with an anti-HA antibody. Ubiquitin and SUMO expression and efficiency of purification were controlled using an anti-6His or anti-SUMO antibody, respectively (bottom). (A–D) Analysis of ubiquitin-conjugated forms of genomically HA-tagged Nup60 was performed in WT and rad6Δ cells (A); WT, slx5Δ, slx8Δ, uls1Δ, and strains (B); WT and ubp10Δ cells (C); or WT and nup60 K105-175R mutants (nup60-Ub-KR; D). (E) Analysis of SUMO conjugated forms of genomically HA-tagged Nup60 was performed in WT and ulp1ts cells grown overnight at permissive temperature (25°C) and then shifted to restrictive temperature (37°C) for 3 h. (F) Nup60 SUMOylation was analyzed in ulp1 ts cells expressing HA-tagged WT and indicated nup60 KR mutants as in D. (G) Position of Nup60 lysines conjugated to ubiquitin or SUMO.