Figure 4.
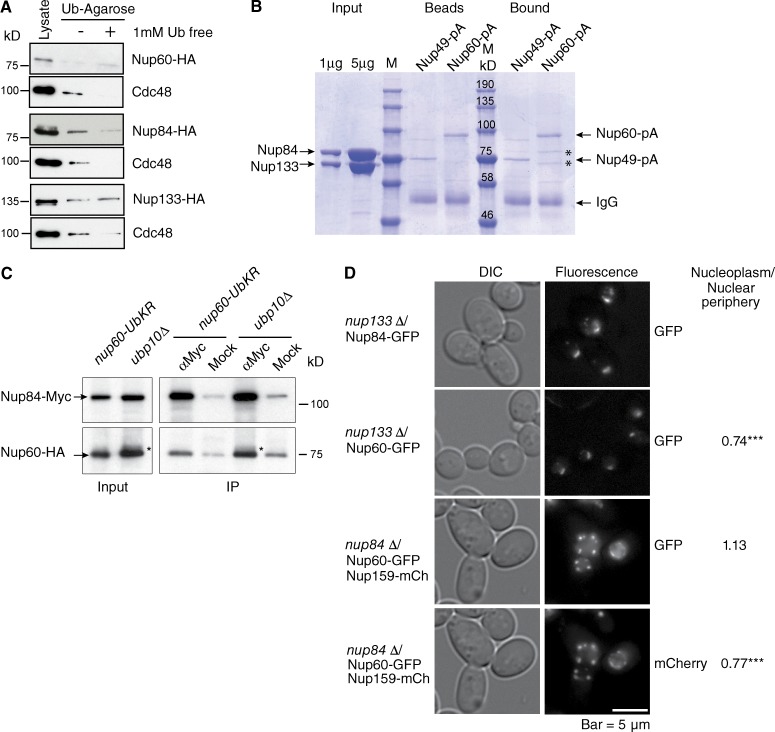
Nup84 interacts with monoubiquitin and is required for tethering Nup60 at the NPC. (A) Lysates from cells expressing HA-tagged Nup60, Nup84, or Nup133 were purified on monoubiquitin-coupled agarose beads in the absence (−) or presence (+) of 1 mM ubiquitin. Bound proteins were analyzed by Western blotting using anti-HA or anti-Cdc48 antibodies. (B) Recombinant purified Nup84 complexed with aa 481–1,157 of Nup133 was purified on Nup49-ProtA (Nup49-pA)- or Nup60-ProtA (Nup60-pA)-coupled IgG beads. Input, Nup-coupled beads and bound material were analyzed by SDS-PAGE and Coomassie blue staining. *, bound Nup84/Nup133. (C) Lysates (Input) from nup60-Ub-KR or ubp10Δ cells expressing Nup60-HA and Nup84-Myc were immunoprecipitated (IP) using anti-Myc or mock antibodies and analyzed by Western blotting with anti-Myc and anti-HA antibodies. *, ubiquitylated form of Nup60 accumulated in ubp10Δ cells. (D) Steady-state localization of GFP-tagged Nup60 and Nup84 in nup133Δ (top), and GFP tagged Nup60 and mCherry tagged Nup159 in nup84Δ cells (bottom). Intensity of Nup60-GFP and Nup159-mCherry was determined both in the nucleoplasm and at the nuclear periphery (total nucleus-nucleoplasm) in nup133Δ (n = 69) and nup84Δ (n = 52) cells using ImageJ, and the nucleoplasm/nuclear periphery ratio was calculated in each cell. Results were compared using Student’s t test (***, P < 0.0001).