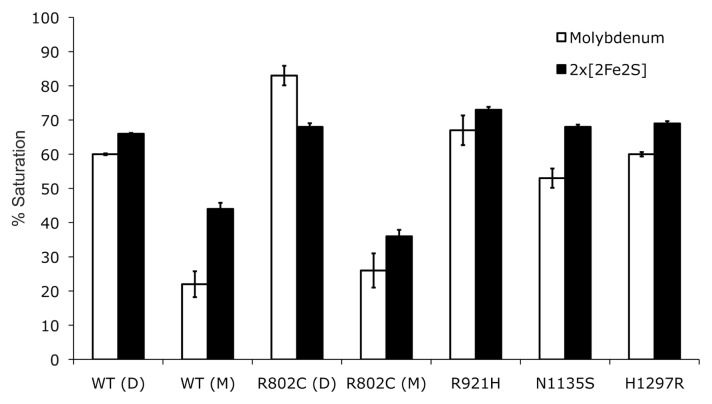
Fig. 2.
Saturation of hAOX1 with molybdenum and iron. Determination of the cofactor saturation of hAOX1 by inductively coupled plasma optical emission spectroscopy. The iron content corresponds to the saturation of both FeSI and FeSII clusters. The wild-type protein and all of its variants show a similar saturation of 2×[2Fe2S] but vary in their molybdenum content saturations between 60 and 80% (D, dimer; M, monomer). The percentage values are related to the theoretical full complement of Moco and the 2×[2Fe2S] clusters.