Abstract
The aim of the present study was to evaluate the independent relationships of systolic blood pressure recovery (SBPR) with age, sex, body mass index (BMI), waist circumference (WC), resting heart rate (HR), physical activity, and cigarette smoking in healthy adults. Subjects performed cycle ergometer exercise at progressive incremental workloads until subjects reached 80% of their age-predicted maximum HR. Blood pressure (BP) was measured before exercise (after 10 and 15 minutes of rest), during exercise (at 2-minute intervals), immediately after exercise (within the first minute) and subsequently at 2-minute intervals until recovery to baseline. The ratio of third-minute SBP relative to first-minute post-exercise SBP was used as the SBPR variable. Our results indicated independent correlations (p<0.05) between SBPR and age, resting HR, physical activity and cigarette smoking (r =0.473; 0.192; −0.262; 0.102 respectively in males and r =0.113; 0.315; −0.637; 0.104 respectively in females). BMI associated positively (r =0.106; p<0.01) with SBPR in males but not in females (r =0.092), while WC was predictive of SBPR in females (r =0.212; p<0.01) but not in males (r =0.005). Age in men and physical activity in females were the strongest predictors of SBPR. The present findings in which SBPR is associated with risk factors of cardiovascular abnormalities strengthen the previously reported significance of SBPR after exercise test as a prognostic tool for the evaluation of cardiovascular abnormalities. Additionally, it may help clinicians to define and interpret the mechanisms behind changes in postexercise SBP responses in adults in future investigations.
Keywords: Ergometer exercise, age, physical activity, body mass index, waist Circumference, resting heart rate, systolic blood pressure ratio
INTRODUCTION
Over the last several years, clinical evaluation of systolic blood pressure recovery (SBPR) as a prognostic tool for diagnosing cardiovascular abnormalities in patients undergoing exercise testing has become the subject of interest (17, 25, 27, 29, 38). In these studies, a delay in SBPR is associated with increased risk of cardiovascular diseases such as coronary artery disease, angina pectoris, hypertension, acute myocardial infarction and stroke. The SBPR is evaluated using a very useful and readily obtainable parameter, the third minute SBP ratio, which is defined as the ratio of SBP in 3 min of recovery relative to either the peak-exercise SBP (41), or SBP in 1 min of recovery (29). However, the third-minute SBP relative to 1 min post-exercise SBP (SBPR2) is often preferred to the third-minute SBP ratio relative to peak-exercise SBP (SBPR1) because it has the advantage of the accuracy of blood pressure measurement (29), since both SBPs can be obtained only in the recovery state. This avoids the inaccuracy associated with exercise blood pressure measurement (12). A value of the third-minute SBP ratio relative to 1 min post-exercise SBP greater than 1.0 is considered a delayed SBP recovery (29).
Since delayed SBPR is a risk factor for cardiovascular diseases, there is a need to evaluate the relationships between SBPR and factors previously associated with cardiovascular events in order to provide better basis for defining and interpreting changes in post-exercise SBP responses to physical stress in future investigations. Age, obesity index, resting heart rate (HR), physical fitness, and cigarette smoking have been associated with SBP response during exercise (6), and reported to be risk factors for hypertension, coronary artery disease and other cardiovascular events (6, 7). In contrast, post-exercise SBPR has been shown to indicate age and gender differences and related to physical fitness and HR during recovery (9, 10, 27). However, no study to our knowledge has associated SBPR with obesity indices (body mass index (BMI) and waist circumference), resting HR or cigarette smoking. Similarly, no study has compared the predictive strength of the above variables on SBPR.
In the present study, we demonstrated whether age, sex, BMI, waist circumference, resting HR, physical activity, and cigarette smoking are independently related to postexercise SBPR or not, in Nigerian adults who performed cycle ergometer exercise. In addition, we determined which of these variables best predicts changes in SBPR.
METHOD
Subjects
Three hundred and thirty seven apparently healthy, normotensive subjects between the ages of 18 to 66 years, selected from students and staff of Igbinedion University, Okada and residents of Okada town in Edo state Nigeria, participated in the study. Of this population, 172 were males and 165 were females. Subjects were randomly selected based on the results of a structured health and lifestyle screening questionnaire, physical examination, morphometric measurements and medical history. Information on cigarette smoking was obtained by verbal report from the subjects. Subjects were classified as smokers or nonsmokers. Physical activity statuses of the subjects were evaluated using the international physical activity questionnaire (IPAQ) for adults (3). The IPAQ comprises a set of 4 questionnaires which are used to assess the physical behaviors of participants at different times and places and the time spent being physically active in the last 7 days. The reliability and validity of IPAQ has been tested and results suggest that it is an acceptable measure of physical activity in adults (3). Based on the IPAQ scores, subjects were classified as inactive, moderately active and highly active. These scores were recorded as categorical data (inactive=1; moderately active=2; highly active=3). Criteria for inclusion in the study were as follows: (a) ability of a subject to perform a vigorous cycle ergometer exercise at 80% of age–predicted maximum HR intensity (b) no prior history of unstable cardiovascular, peripheral vascular and respiratory disease, malignancy, and orthopedic or musculoskeletal disorders (c) subjects should be nonobese, and non-diabetics (d) subjects should have normal blood pressure (BP) and HR (e) not taking medications that could affect cardiovascular functions (f) not menstruating at the time of test if female. Subjects were informed (written and oral) of the experimental procedures and their consents were obtained before participation. The Experiments and Ethics Committee of the College of Health Sciences of the Igbinedion University Okada, Edo State approved the study.
Exercise Test
The exercise tests were carried out between 8.00 AM and 11.00 AM in a well-ventilated room, using a mechanically braked cycle ergometer (Homeware Ltd, North York, Ontario, Canada). With the ergometer cycling protocol, it is easy to obtain reliable blood pressure measurements especially during recovery period. The cycle ergometer usually consists of progressive incremental workloads that may have minor effects on SBPs achieved during the exercise test (27). Participants were instructed not to consume beverages containing alcohol or coffee, not to eat a heavy meal, or participate in any vigorous physical activity 24 hours before the test. They were also properly instructed on how to perform the exercise test with demonstrations. The exercise protocol comprised an initial two-minute warm up at a work load of 20 Watts, followed by a linear increase of 20 Watts every minute until the subject reached the targeted percentage (80%) of age-predicted maximum HR (HRmax), after which the exercise test was terminated. The HRmax was determined as [HRmax = 208 minus (0.7 × age)], (40). The rating of perceived exertion (RPE) to exercise was obtained using the Borg’s scale (2) immediately after the exercise protocol.
Anthropometric Measurements
Subject’s height was measured to the nearest 0.1 cm with the use of stadiometer (SECA, Hamburg, Germany) with the shoulders in a relaxed position and the arms hanging freely. Weight was measured to the nearest 0.1 kg in light clothing without shoes using a balance scale. BMI was calculated as weight (kg) divided by the square of the height (m2). Waist circumference was measured twice to the nearest 0.1 cm using an inelastic and flexible tape, on a horizontal plane at the end of normal expiration with subjects lightly clothed and standing. The mean of the two measurements was used for subsequent analysis. Waist circumference was measured half way between the top of the iliac crest and the lower rib margin.
Blood Pressure and Heart Rate Measurements
Resting BP and HR were measured after 10 and 15 minutes of rest, in a seated position and in a quiet room, one week prior to the exercise test, using the mercury-column sphygmomanometer and an automated upper arm-cuff HR monitor (HEM-712, Omron Health Care Inc., Vernon Hills, Illinois) respectively. The resting BP and HR measurements were used to ascertain whether a subject had normal BP and HR or not. Immediately before the exercise test, subject’s pre-exercise BP and HR were also measured twice (after 10 and 15 minutes of rest) when sitting on the cycle ergometer. During the exercise, BP was measured at two-minute intervals and during the last minute of exercise, as soon as the subject reached his or her targeted HR. Heart rate on the other hand, was measured continuously every minute until the subject attained the targeted HR equivalent to the 80% HRmax intensity. The peak-exercise BP and HR were defined as the highest values achieved at the termination of the exercise. Further BP measurements were done immediately after exercise (within the first minute of recovery) and subsequently at two-minute intervals until recovery to pre-exercise level (i.e. measurements were done at 1, 3, 5, 7,…min). During the post-exercise BP measurement, subjects were asked to be in sitting position on the bicycle without pedaling while the research personnel were blinded to the test results at baseline and during exercise. First and third minutes of recovery were used to express the periods of recovery after exercise in this study, since they were available for all subjects. We evaluated SBPR using the ratio of third minute SBP relative to SBP at 1 min of recovery (29), calculated as 3 min postexercise SBP divided by 1 min post-exercise SBP. We preferred SBPR2 as our index of SBPR because both SBPs are obtained only in the recovery state, thus avoiding the inaccuracy associated with exercise blood pressure measurement (12).
Statistical Analyses
Descriptive data are presented as means ± SD. Data analyses between the gender groups were performed using the independent sample t-test. Pearson’s bivariate correlation test was used to evaluate the relationships between SBPR2 and age, BMI, WC, resting HR, physical activity status, and cigarette smoking. Independent relationships between SBPR2 and the predictor variables were analyzed using multiple linear regression with the SBPR2 as the dependent variable. All statistics were done using SPSS for Windows (Version 16.0). Statistical significance was set at p<0.05.
RESULTS
Demographic data and baseline characteristics of subjects are as presented in table 1. The mean ages of the subjects were 28 ± 13.95 and 27 ± 14.73 years for the males and the females respectively. Waist circumference, pre-exercise SBP and pre-exercise DBP were significantly higher in males than females. On the other hand, females indicated higher resting HR than the males. Body mass index indicated no significant difference between the genders.
Table 1.
Demographic and baseline characteristics of subjects.
| CHARACTERISTICS | MALES (n=172) | FEMALES (n=165) | P-value |
|---|---|---|---|
| Age (yrs) | 28.0 ± 13.95 (18–66) | 27.0 ± 14.73 (18–65) | NS |
| Height (m) | 1.7 ± 0.07 (1.5–1.9) | 1.6 ± 0.06 (1.5–1.8) | <0.001 |
| Weight (kg) | 66.7 ± 8.48 (51–87) | 60.3 ± 9.05 (43–86) | <0.001 |
| BMI (kg/m2) | 21.9 ± 8.31 (18.1–28.0) | 21.7 ± 7.75 (18.0–27.2) | NS |
| Waist Circumference (cm) | 80.4 ± 7.35 (70–94) | 74.8 ± 6.75 (68–82) | <0.001 |
| Pre-exercise SBP(mmHg) | 120.0 ± 8.41 (99–136) | 118.0 ± 10.26 (96–137) | <0.05 |
| Pre-exercise DBP(mmHg) | 78.0 ± 6.05 (66–88) | 74.0 ± 7.79 (60–88) | <0.001 |
| Resting HR (bpm) | 73.0 ± 9.57 (58–84) | 75.0 ± 6.29 (63–81) | <0.05 |
Data are means ± SD and range. Abbreviations: n= number of subjects; NS, not significant; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate.
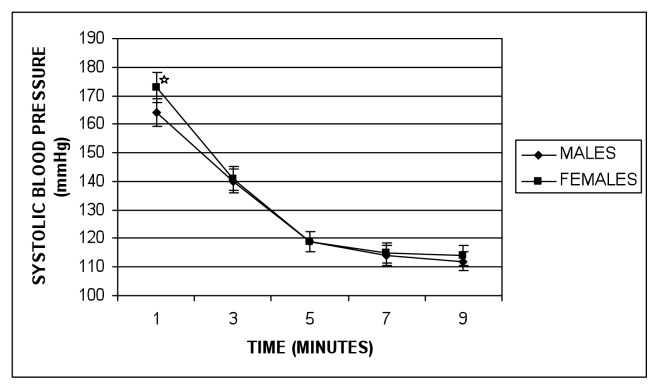
Table 2 shows the exercise test characteristics of subjects. Men indicated significantly higher peak-exercise SBP, peak-exercise DBP, SBPR1, and SBPR2 than did the women. Peak exercise HR and rating of perceived exertion indicated no significant differences between the genders. Changes of SBP as it falls from peak-exercise to baseline during recovery are as shown in figure 1. At 1 minute of recovery, SBP declined to 164 mmHg for males, and 173 mmHg for females, while at 3 minutes of recovery, the data indicated 140 mmHg and 141 mmHg for males and females respectively.
Table 2.
Exercise test characteristics of subjects.
| CHARACTERISTICS | MALES (n=172) | FEMALES (n=165) | P-value |
|---|---|---|---|
| Peak-Exercise SBP (mmHg) | 184 ±11.31 (150–210) | 180 ± 13.00 (144–197) | <0.001 |
| Peak-Exercise DBP (mmHg) | 81.0 ± 6.00 (69–91) | 75.0 ± 7.62 (60–90) | <0.001 |
| Peak Exercise HR (bpm) | 151.0 ± 7.75 (129–156) | 150.0 ± 8.89 (129–156) | NS |
| RPE | 16.8 ± 0.65 (16–18) | 16.8 ± 0.70 (16–18) | NS |
| SBPR1 | 0.76 ± 0.04 (0.72–0.90) | 0.74 ± 0.08 (0.57–0.91) | <0.05 |
| SBPR2 | 0.85 ± 0.03 (0.76–0.94) | 0.81 ± 0.08 (0.62–0.95) | <0.001 |
Data are means ± SD and range. Abbreviations: n= number of subjects, NS= not significant; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; RPE, rating of perceived exertion; SBPR1, third minute systolic blood pressure relative to peak exercise; SBPR2, third minute systolic blood pressure relative to 1 min of recovery.
Figure 1.
Changes of SBP during recovery periods in males and females. * = Significant gender difference (p<0.001).
Table 3 shows a bivariate correlation analysis between SBPR2 and age, BMI, waist circumference, resting HR, physical activity status, and cigarette smoking in males and females. In both gender groups, all variables indicated significant and positive relationships with SBPR2 except physical activity status, which was negatively associated with SBPR2.
Table 3.
Bivariate correlation coefficients and p values for the association of predictor variables with SBPR2 in males and females.
| Males | Females | |||
|---|---|---|---|---|
| Coefficient | P-value | Coefficient | P-value | |
| Age | 0.895 | <0.001 | 0.305 | <0.05 |
| BMI | 0.747 | <0.001 | 0.486 | 0.355 |
| Waist Circumference | 0.387 | <0.001 | 0.551 | 0.969 |
| Resting Heart Rate | 0.915 | <0.001 | 0.743 | <0.01 |
| Physical activity level | −0.860 | <0.001 | −0.857 | <0.001 |
| Smoking | 0.620 | <0.001 | 0.250 | <0.001 |
Table 4 reveals a multiple regression analysis to evaluate the independent relationship between SBPR2 and age, BMI, waist circumference, resting HR, physical activity status, and cigarette smoking in males and females. In men, all the variables except waist circumference remained significantly predictive of SBPR2 after adjusting for each other. In women, SBPR2 associated significantly with all the variables except BMI.
Table 4.
Multiple linear regression coefficients and p values for the association of predictor variables with systolic blood pressure in males and females.
| Males | Females | |||
|---|---|---|---|---|
| Coefficient | P-value | Coefficient | P-value | |
| Age | 0.473 | <0.001 | 0.113 | <0.01 |
| BMI | 0.106 | <0.01 | 0.092 | 0.146 |
| Waist Circumference | 0.005 | 0.823 | 0.212 | <0.01 |
| Resting Heart Rate | 0.192 | <0.01 | 0.315 | <0.001 |
| Physical Activity Level | −0.262 | <0.001 | −0.637 | <0.001 |
| Smoking | 0.102 | <0.001 | 0.104 | <0.01 |
Combining the data for men and women, we evaluated the independent association between gender and SBPR. A multiple regression analysis indicated a significant and independent relationship between gender and SBPR2 (r = 0.248; p<0.001).
DISCUSSION
Our findings indicated that, in both genders, age, resting HR, and cigarette smoking were independently and positively associated with SBPR, while physical activity indicated negative association with SBPR; body mass index, and waist circumference were predictive of SBPR in at least one gender-specific group. Age in males and physical activity in females were the best predictors of SBPR.
Changes in SBPR are thought to be due to changes in systemic vascular resistance (25, 41), sympathetic and parasympathetic activities (25, 27), and baroreflex sensitivity (34). Similarly, SBPR has been reported to indicate age and gender differences and related to physical fitness and HR recovery (9, 10, 27). Blunted or delayed recovery of SBP after exercise is also associated with increased risk of cardiovascular diseases (17, 25, 27, 29, 38).
In the present study, age was independently and positively related to SBPR in males and females. Age has been previously associated with blood pressure responses (12). Age differences have also been found in SBPR after exercise with the older subjects indicating slower SBP recovery than younger adults (10). Changes observed in blood pressure responses due to advancing age are suggested to be due to increase in systemic vascular resistance (26, 31), decrease in parasympathetic activity (8), elevated sympathetic activity (37), decline in physical fitness (14), and reduced baroreflex sensitivity (22) in older adults. Ageing is also related to increased risk of cardiovascular diseases (26, 31). These factors as already stated above are associated with SBPR after exercise and may help explain the independent relationship observed between age and SBPR in the present study.
Overweight and obesity have been previously related to blood pressure response during exercise (4) but not to postexercise SBPR. Previous studies (23, 24) have consistently shown that both absolute total fat and adipose tissue distribution are closely associated with the risk of diabetes, hypertension, hyperlipidaemia and cardiovascular diseases. BMI appears to be the best and most cited index for obesity because it approximates adiposity and fat distribution in adults (36). It is also considered a strong predictor of metabolic risks (39). Recent studies have also suggested that waist circumference is the best index of abdominal visceral adipose tissue (33) and may also be the best index for predicting cardiovascular risks (35).
The present data demonstrated independent and positive relationships between SBPR and BMI in males, and waist circumference in females. These findings show that an increase in level of adiposity will lead to increase in SBPR2 (slower SBPR) and also suggest that BMI was the better obesity index that explained variations in SBPR in males while waist circumference best predicted SBPR in females. Our findings concur with previous studies which have shown that central obesity is more closely associated with cardiovascular risks than general obesity in women (19, 30), while general obesity best predicts cardiovascular risks in men (19).
The present data indicated that the SBP recovery ratios consistently related positively to resting HR in both genders. This indicates that a low resting HR will result in faster SBPR and vice versa. No previous study to our knowledge has associated resting HR to SBPR. The present result however was expected since changes in blood pressure are usually mediated by the baroreflex mechanism via HR changes (13). The baroreflex mediated response of HR to changes in arterial blood pressure indicates the capacity of reflex cardiac autonomic modulation (20). Furthermore, low resting HR has been reported to be a partial surrogate for good conditioning and regular exercise (6) and reflects good health (1), whereas higher values are related to higher cardiovascular mortality (15). Similarly, faster SBPR after exercise has been previously related to higher physical activity and fitness level (27), while a delayed (slower) SBPR is associated with increased risk of cardiovascular diseases.
The level of physical activity has been previously associated with SBP responses to exercise (32) and generally regarded as a very important risk factor for cardiovascular diseases. The rate at which SBP declines after exercise is suggested to be a reflection of a person’s level of physical activity and fitness; a more rapid decline indicates a higher level of physical fitness, and a greater decrease in SBP from peak exercise to the recovery may reflect good aerobic capacity (27). In the present study, a higher physical activity level of subjects was associated with faster SBPR and consistent with the previous studies. The mechanisms behind the observed relationship between physical activity and SBPR are not very clear. However, this may be connected with the effect of exercise training in improving vascular endothelial functions and vasodilatory capabilities, hence a decrease in systemic vascular resistance (16, 28).
Cigarette smoking has been shown to increase blood pressure and HR, decrease exercise tolerance and is associated with cardiovascular diseases such as coronary heart disease, stroke, and peripheral vascular diseases (18). However no study has associated cigarette smoking with SBPR. In this study, cigarette smoking indicated independent and positive associations with delayed SBP recovery in both genders. Smokers showed 0.10 higher SBPR2 than non-smokers in both genders. These results may be a reflection of poor response of SBP to exercise in poorly conditioned smokers. The mechanism by which smoking slows down SBP recovery is not well understood, but it is thought that smoking through the activities of nicotine and carbon monoxide contributes to the aggravation and acceleration of arterial wall stiffness and inelasticity (18), thus increasing systemic arterial resistance.
In order to determine the relationship between gender and SBPR, we combined the data for men and women to perform a multiple regression analysis adjusting for all the other variables. Our data showed that men showed 0.25 higher SBPR2 than women. This result indicates that women demonstrated faster SBPR than men and inconsistent with our previous study (9). Previous studies (25, 27) have suggested that SBP recovery will be delayed with increased sympathetic activity and attenuated vagal reactivation. It has also been reported that at all ages women have been found to have reduced sympathetic activity and enhanced parasympathetic activity relative to men (21). Epidemiological studies have also demonstrated a gender difference in the incidence of cardiovascular disease, with women, particularly younger women, at much lower risk of developing cardiovascular disease than their age-matched men (5). These facts support our present findings in which women indicated faster SBPR than males.
It is noteworthy that among all the variables studied, age in men and physical activity in women indicated the strongest associations with SBPR. Previous studies (11, 27) have demonstrated the importance and influence of age and physical activity on SBPR but none to our knowledge has compared the strength of associations of predictive variables with SBP recovery. The present findings therefore may suggest that age in men and physical activity status in females should be given more importance when evaluating changes in SBP recovery and during screening of associated cardiovascular events. Further studies are however needed to strengthen these findings.
Limitations of study: Our study involved adults who performed ergometer exercise tests at a submaximal level (vigorous exercise intensity). In addition, we evaluated systolic blood pressure recovery during inactive exercise recovery mode. Our study therefore may not apply to SBP recovery from other exercise types and intensities; or to other cycling exercise recovery modes. Further studies are therefore recommended in these areas.
In summary, the present study indicated independent relationships between SBP recovery and variables known to associate with cardiovascular abnormalities such as age, BMI and waist circumference, resting HR, physical activity, and cigarette smoking in at least one gender-specific group of apparently healthy adults. These findings strengthen the previously reported prognostic importance of post-exercise SBP recovery in diagnosing cardiovascular abnormalities in healthy adults undergoing exercise stress tests. Additionally, it will provide a better basis on which to define and interpret the mechanisms behind changes in post-exercise SBP responses in healthy adults undergoing stress tests in future investigations.
REFERENCES
- 1.Almeida MB, Araujo CGS. Effects of aerobic training on heart rate. Rev Bras Med Esporte. 2003;9(2):113–120. [Google Scholar]
- 2.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exc. 1982;14:377–381. [PubMed] [Google Scholar]
- 3.Booth ML. Assessment of physical activity: an international perspective. Res Q Exerc Sports. 2000;71(2):s114–20. [PubMed] [Google Scholar]
- 4.Carletti L, Rodrigues AN, Perez AJ, Vassalo DV. Blood pressure response to physical exertion in adolescents; influence of overweight and obesity. Arq Bras Cardiol. 2008;91:25–30. doi: 10.1590/s0066-782x2008001300004. [DOI] [PubMed] [Google Scholar]
- 5.Castelli WP. Epidemiology of coronary heart disease: the Framingham study. Am J Med. 1984;76:4–12. doi: 10.1016/0002-9343(84)90952-5. [DOI] [PubMed] [Google Scholar]
- 6.Criqui MH, Haskell WL, Heiss G, Tyroler HA, Green P, Rubenstein CJ. Predictors of systolic blood pressure response to treadmill exercise: the Lipid Research Clinics Program Prevalence Study. Circulation. 1983;68:225–233. doi: 10.1161/01.cir.68.2.225. [DOI] [PubMed] [Google Scholar]
- 7.Daida H, Allison TG, Squires RW, Miller TD, Gau GT. Peak exercise blood pressure stratified by age and gender in apparently healthy subjects. Mayo Clin Proc. 1996;71:445–452. doi: 10.4065/71.5.445. [DOI] [PubMed] [Google Scholar]
- 8.Davy KP, DeSouza CA, Jones PP, Seals DR. Elevated heart rate variability in physically active young and older adult women. Clinical Science. 1998;94:579–584. doi: 10.1042/cs0940579. [DOI] [PubMed] [Google Scholar]
- 9.Dimkpa U, Ugwu AC, Oshi DC. Assessment of sex differences in systolic blood pressure responses to exercise in healthy, non-athletic young adults. JEPonline. 2008;11(2):18–25. [Google Scholar]
- 10.Dimkpa U, Ugwu AC. Age-related differences in systolic blood pressure recovery after a maximal effort exercise test in non-athletic adults. Int J Exerc Sci. 2008;1(4):142–152. [PMC free article] [PubMed] [Google Scholar]
- 11.Dimkpa U, Ugwu AC. Influence of age on blood pressure recovery after maximal effort ergometer exercise in non-athletic adult males. Eur J Appl Physiol. 2009;106(6):791–797. doi: 10.1007/s00421-009-1081-y. [DOI] [PubMed] [Google Scholar]
- 12.Ellestad MH. Stress testing: Principles and practice. 3rd Ed. Philadelphia: FA Davis; 1986. pp. 472–474. [Google Scholar]
- 13.FitPro TC. Blood pressure and exercise. American Fitness Professionals and Associates; 1998. Retrieved on 6th April, 2009 from http://www.afpafitness.com/articl/article-andnewsletter/ [Google Scholar]
- 14.Fleg JL, Morell CH, Bos AG, Brant LJ. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 15.Greenland P, Daviglus ML, Dyer AR, Liu K, Huang CF, Goldberger JJ, et al. Resting heart rate is a risk factor for cardiovascular and noncardiovascular mortality: the Chicago Heart Association Detection Project in Industry. Am J Epidemiol. 1999;149:853–862. doi: 10.1093/oxfordjournals.aje.a009901. [DOI] [PubMed] [Google Scholar]
- 16.R, Wolf A, Gielen S, Linke A, Hofer J, Erbs S, et al. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med. 2000;342:454–460. doi: 10.1056/NEJM200002173420702. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto M, Okamoto M, Yamagata T, Yamane T, Watanabe M, Tsuchioka Y, et al. Abnormal systolic blood pressure during exercise recovery in patients with angina pectoris. J Am Coll Cardiol. 1993;22:659–664. doi: 10.1016/0735-1097(93)90173-x. [DOI] [PubMed] [Google Scholar]
- 18.Health-cares.net; your fitness guides. Smoking and cardiovascular disease. Retrieved on 9th June 2009 from http://menshealth.healthcares.net/smokingcardiovasculardisease.php.
- 19.Ho SC, Chen YM, Woo JLF, Leung SSF, Lam TH, Janus ED. Association between simple anthropometric indices and cardiovascular risk factors. Int J Obes. 2001;25:1689–1697. doi: 10.1038/sj.ijo.0801784. [DOI] [PubMed] [Google Scholar]
- 20.Huikuri HV, Pikkujamsa SM, Airaksinen KE, Ikaheimo MJ, Rantala AO, Kauma H, et al. Sex related differences in autonomic modulation of heart rate in middleaged subjects. Circulation. 1996;94:122–125. doi: 10.1161/01.cir.94.2.122. [DOI] [PubMed] [Google Scholar]
- 21.Huxley VH. Sex and the cardiovascular system: the intriguing tale of how women and men regulate cardiovascular function differently. Advan Physiol Edu. 2007;31:17–22. doi: 10.1152/advan.00099.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones PP, Christou DD, Jordan J, Seals DR. Baroreflex buffering is reduced with age in healthy men. Circulation. 2003;107:1770–1774. doi: 10.1161/01.CIR.0000057811.86187.88. [DOI] [PubMed] [Google Scholar]
- 23.Jousilahti P, Toumilehto J, Vartiainen E, Pekkanen J, Puska P. Body weight, cardiovascular risk factors, and coronary mortality. 15 year follow - up of middleaged men and women in eastern Finland. Circulation. 1996;93:1372–1379. doi: 10.1161/01.cir.93.7.1372. [DOI] [PubMed] [Google Scholar]
- 24.Kannel WB, Cupples LA, Ramaswami R, Stokes J, Kreger BE, Higgins M. Regional obesity and risk of cardiovascular disease; the Framingham Study. J Clin Epidemiol. 1991;44:183–190. doi: 10.1016/0895-4356(91)90265-b. [DOI] [PubMed] [Google Scholar]
- 25.Kurl S, Laukkanen JA, Rauramaa R, Lakka TA, Sivenius J, Salonen JJ. Systolic blood pressure response to exercise stress test and risk of stroke. Stroke. 2001;32:2036–2041. doi: 10.1161/hs0901.095395. [DOI] [PubMed] [Google Scholar]
- 26.Lakatta EG. Changes in cardiovascular function with aging. Eur Heart J. 1990;11(Suppl C):22–29. doi: 10.1093/eurheartj/11.suppl_c.22. [DOI] [PubMed] [Google Scholar]
- 27.Laukkanen JA, Kurl S, Salonen R, Lakka TA, Rauramaa R, Salonen JT. Systolic blood pressure during recovery from exercise and the risk of acute myocardial infarction in middle aged men. Hypertension. 2004;44:820–825. doi: 10.1161/01.HYP.0000148460.95060.f2. [DOI] [PubMed] [Google Scholar]
- 28.Mackey RH, Sutton-Tyrell K, Vaitkevicius PV, Sakkinen PA, Lyles MF, Spurgeon HA, et al. Correlates of aortic stiffness in elderly individuals: a subgroup of the Cardiovascular Health Study. Am J Hypertens. 2002;15:16–23. doi: 10.1016/s0895-7061(01)02228-2. [DOI] [PubMed] [Google Scholar]
- 29.McHam SA, Marwick TH, Pashkow FJ, Lauer MS. Delayed systolic blood pressure recovery after graded exercise: an independent correlate of angiographic coronary disease. J Am Coll Cardiol. 1999;34:754–759. doi: 10.1016/s0735-1097(99)00269-7. [DOI] [PubMed] [Google Scholar]
- 30.Mueller WH, Wear ML, Hanis CL, Emerson JB, Barton SA, Hewett-Emmett D, et al. Which measure of fat distribution is best for epidemiologic research? Am J Epidemiol. 1991;133:858–869. doi: 10.1093/oxfordjournals.aje.a115966. [DOI] [PubMed] [Google Scholar]
- 31.Oxeham H, Sharpe N. Cardiovascular aging and heart failure. Eur J Heart Fail. 2003;5(4):427–434. doi: 10.1016/s1388-9842(03)00011-4. [DOI] [PubMed] [Google Scholar]
- 32.Perini R, Orizio C, Comande A, Castellano M, Beschi M, Veicsteinas A. Plasma norepinephrine and heart rate dynamics during recovery from submaximal exercise in man. European Journal of Applied Physiology. 1989;58:879–883. doi: 10.1007/BF02332222. [DOI] [PubMed] [Google Scholar]
- 33.Pouliot MC, Despres JP, Lemieux S, Moorjani S, Bouchard C, Tremlay A, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indices of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–468. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- 34.Raven PB, Potts JT, Shi X. Baroreflex regulation of blood pressure during dynamic exercise in humans. Exerc Sport Sci Rev. 1997;25:365–389. [PubMed] [Google Scholar]
- 35.Reeder BA, Senthilselvan A, Despres JP, Angel A, Liu L, Wang H, et al. The association of cardiovascular disease risk factors with abdominal obesity in Canada. Canadian Heart Health Surveys Research Group. CMAJ. 1997;157(Suppl 1):S39–45. [PubMed] [Google Scholar]
- 36.Sakurai M, Miura K, Takamura T, Ota T, Ishizaki M, Morikawa Y, et al. Gender differences in the association between anthropometric indices of obesity and blood pressure in Japanese. Hypertens Res. 2006;29:75–80. doi: 10.1291/hypres.29.75. [DOI] [PubMed] [Google Scholar]
- 37.Seals D, Esler M. Human ageing and the sympathoadrenal system. J Physiol. 2000;528(3):407–417. doi: 10.1111/j.1469-7793.2000.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh JP, Larson MG, Manolio TA, O’Donnell CJ, Lauer M, Evans JC, et al. Blood pressure response during treadmill testing as a risk factor for a new-onset hypertension. The Framingham heart study. Circulation. 1999;99:1831–1836. doi: 10.1161/01.cir.99.14.1831. [DOI] [PubMed] [Google Scholar]
- 39.Spiegelman D, Israel RG, Bouchard C, Willett WC. Absolute fat mass, percent body fat, and body fat distribution: which is the real determinant of blood pressure and serum glucose? Am J Clin Nutr. 1992;55:1033–1044. doi: 10.1093/ajcn/55.6.1033. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka H, Monahan KD, Seals DR. Age-predicted maximum heart rate revisited. J Am Coll Cardiol. 2001;37(1):153–156. doi: 10.1016/s0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- 41.Taylor AJ, Beller GA. Post-exercise systolic blood pressure response; clinical application to the assessment of ischemic heart disease. American Family Physicians. 1998;58(5):1–9. [PubMed] [Google Scholar]