Significance
Removing megafauna from contemporary ecosystems changes vegetation and small mammal communities over ecological time scales. We show that similar dynamics seem to operate over millennial time scales but only if the megafaunal loss includes ecosystem engineers in settings that also contain plant species susceptible to ecological release. Under such conditions, megafauna extinction can initiate changes that quickly lead to new, lasting ecological states. This implies that should some megafauna currently at risk for extinction actually become extinct, their characteristic ecosystems—for example, mosaics of savannah and forest—would also disappear, rapidly transforming into novel systems with respect to what is considered normal in today’s world.
Keywords: megafauna, extinction, Quaternary, North America, South America
Abstract
Loss of megafauna, an aspect of defaunation, can precipitate many ecological changes over short time scales. We examine whether megafauna loss can also explain features of lasting ecological state shifts that occurred as the Pleistocene gave way to the Holocene. We compare ecological impacts of late-Quaternary megafauna extinction in five American regions: southwestern Patagonia, the Pampas, northeastern United States, northwestern United States, and Beringia. We find that major ecological state shifts were consistent with expectations of defaunation in North American sites but not in South American ones. The differential responses highlight two factors necessary for defaunation to trigger lasting ecological state shifts discernable in the fossil record: (i) lost megafauna need to have been effective ecosystem engineers, like proboscideans; and (ii) historical contingencies must have provided the ecosystem with plant species likely to respond to megafaunal loss. These findings help in identifying modern ecosystems that are most at risk for disappearing should current pressures on the ecosystems’ large animals continue and highlight the critical role of both individual species ecologies and ecosystem context in predicting the lasting impacts of defaunation currently underway.
Defaunation is occurring at a rapid pace presently (1–3). Losses are particularly severe for megafauna (1) (considered here as animals with an average body size ≥44 kg), whose removal can trigger the following: changes in vegetation structure and species composition; reductions in environmental heterogeneity, species richness, evenness, seed dispersal, nutrient cycling and distribution, and ecosystem services; coextinction of dependent species; and increases in disease-transmitting organisms (1, 4–14) and fire frequency and/or intensity (15–17).
Most work on defaunation has been in contemporary ecosystems. Much less is known about how it manifests over millennial time scales. A natural experiment to assess lasting effects of megafauna loss is provided by the extinctions of late-Quaternary megafauna in the Americas, part of global-scale ecological state shift (18), during which about half of the world’s large-bodied mammal species (19, 20) disappeared. In North America, ∼60 megafaunal species died out, with the youngest occurrences of dated species typically falling between ∼13,000 and 11,000 y ago (19). In South America, ∼66 species were lost over a longer time span (21–23).
With a few important exceptions (6, 17, 24–29), the major changes in vegetation and mammalian community structure that accompanied Quaternary extinctions have been interpreted as responses to changing climate (17–19, 21, 23, 25–27, 29–35). Here, we build on recent work of paleoecologists (17, 25, 28, 29, 32, 36) and ecologists (1, 3–7, 9, 10, 15, 16, 37) who have been asking instead: Are the observed biotic responses consistent with megafauna loss, and if so, what does this loss imply for the future of ecosystems at risk for losing their megafauna today?
Approach
The late-Quaternary impact of losing 70–80% of the megafauna genera in the Americas (19) would be expected to trigger biotic transitions that would be recognizable in the fossil record in at least two respects. First, vegetation should change noticeably, consistent with ecological release from browsing, grazing, and trampling; such changes should be apparent in fossil-pollen time series (17, 25, 29) and possibly also in charcoal (fire-frequency) records (15–17, 36). Second, surviving mammal communities should demonstrate changes in species composition, richness, and evenness (32). Testing for such impacts requires fossil sites with the following: (i) good records of extinct megafauna; (ii) proximal and temporally overlapping records of vegetation change and fire frequency; (iii) associated records of species that allow assessment of mammalian diversity; (iv) robust dating of megafauna population crashes and extinction and vegetation, fire, and diversity changes; and (v) a fossil record that temporally samples before and after the extinctions.
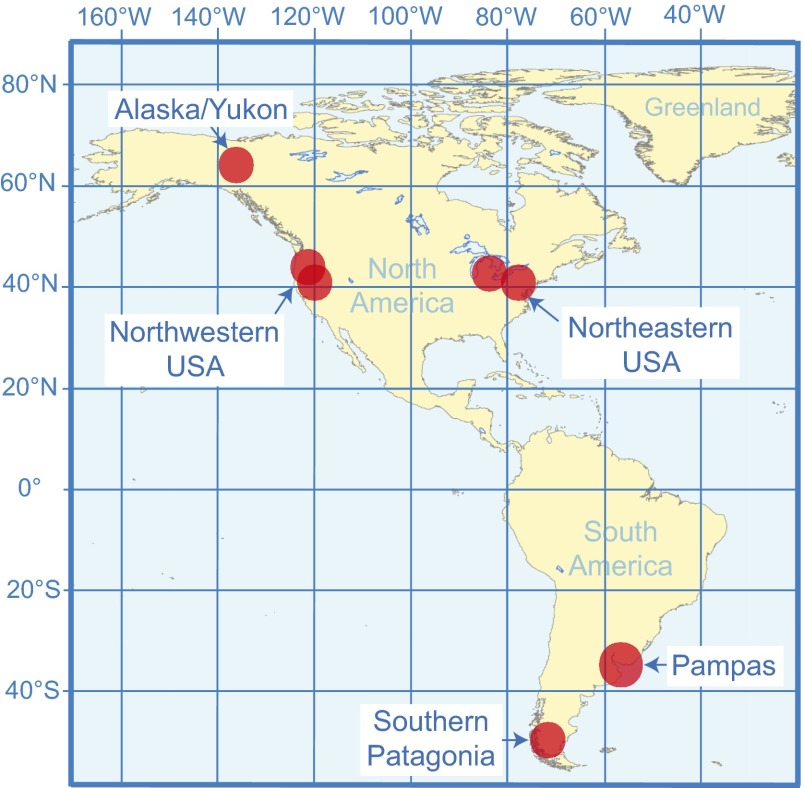
Here, we examine evidence from five regions where paleontological records are adequate in at least most of these respects: southwestern Patagonia, the Pampas, northeastern United States, northwestern United States, and the Alaska/Yukon area (Beringia) (Fig. 1). Each region featured different megafauna and/or vegetation, and each provides relatively rich datasets within constrained biogeographic settings (see Supporting Information for the South American sites). If defaunation triggers ecological state shifts universally, in all areas, we would expect to see as megafauna go extinct: (i) increases in understory and forest plants susceptible to ecological release in the absence of herbivory, trampling, and related pressures, potentially with increased fire frequency; and (ii) predictable changes in surviving communities of mammal species, such as adjustments in geographic range, species density, and diversity. Disentangling defaunation impacts from those triggered by end-Pleistocene climate change requires observing a discordance between the timing of defaunation and climate change proxies with respect to the hypothesized biotic responses, and/or by observing biotic indicators (for example, of vegetation change or fire frequency) that make more sense as the result of a defaunation rather than from a climatic trigger. The relative contribution of human activity and climate change in causing the late-Quaternary defaunation events is still debated (19). However, our goal here is simply to understand whether or not the chronology of megafauna extinction, changes in vegetation and fire records, and changes in mammal species diversity support or reject the expectations of ecological changes triggered by megafauna extinction, regardless of the ultimate cause of defaunation. We emphasize that, given the limitations of the fossil record, only the coarsest effects of defaunation—such as vegetation and diversity changes we examine here—can be recognized by our approach.
Fig. 1.
Areas examined for impacts of late-Quaternary defaunation.
Vegetational Changes
South America—Southwestern Patagonia.
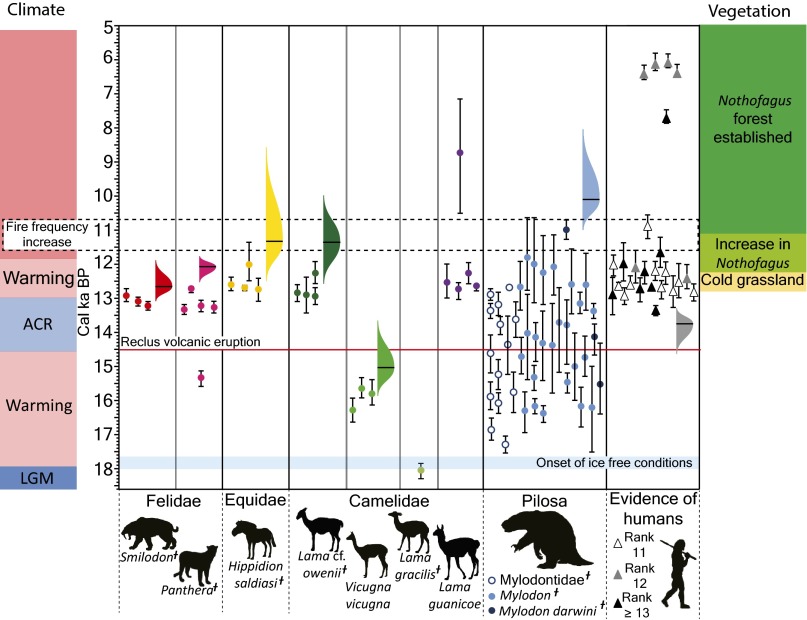
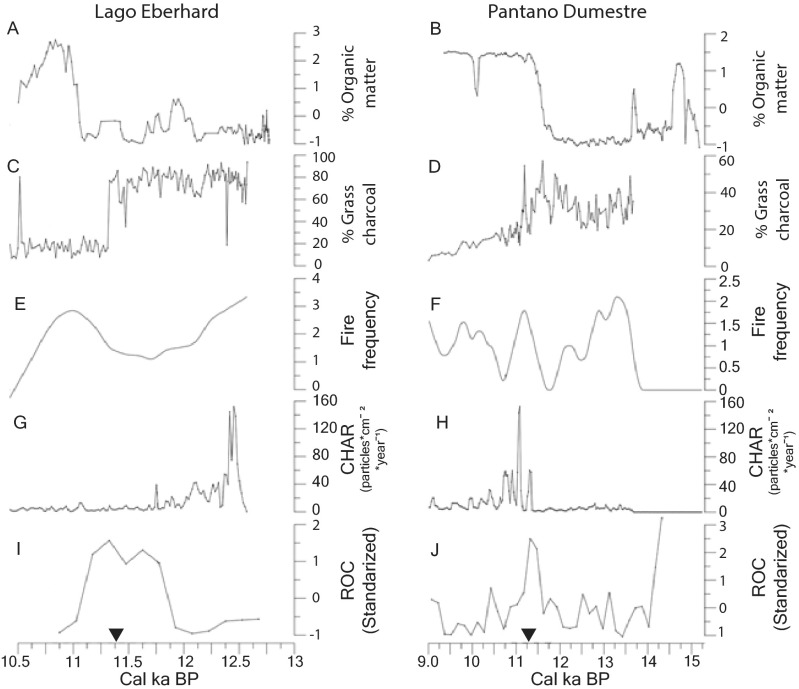
Recent work in the Última Esperanza region in Chile has produced a detailed chronology of colonization of the region by humans and megafauna, megafauna extinction, and vegetation, fire, and climatic changes for the period 19,000 y ago to 5,000 y ago (23) (Figs. S1–S4). There, Lama gracilis and Vicugna vicugna [these two taxa may be synonymous (38)] became locally extinct about 15,000 y ago. Humans arrived in the area by 13,750 y ago. The extinction of megacarnivores (Smilodon and Panthera), ∼1,100 and 1,700 y later, respectively, occurred just after the Antarctic Cold Reversal, during the beginning of climatic warming that apparently initiated a shift from cold grassland vegetation to Nothofagus forest (23, 39) (Fig. S4). Hippidion saldiasi and a llama (Lama cf. L. owenii of past publications) disappeared ∼2,400 y later, as transition to Nothofagus continued. At about the same time, fire frequency also increased, consistent with warming temperatures and increased fuel provided by the encroachment of trees. It may be that extinction of the equid and llama also helped to increase fire fuels (Fig. S4) and promote expansion of the Nothofagus forests, which had arrived in the region at least 500 y earlier (Figs. S2–S4). By ∼11,000 y ago, Nothofagus forests were firmly established, and it is only after that (most likely near ∼10,100 y ago) that Mylodon darwini goes extinct. The overall pattern of herbivore extinction (Fig. S4) is equivocal with respect to defaunation triggering some of the observed vegetation and fire changes (23). Although loss of Hippidion and L. cf. L. owenii potentially corresponds with increasing forest cover and fire frequency, these changes also could be explained entirely by contemporaneous climate changes in the region. The extinction of Mylodon does not correlate with any major vegetation or fire changes and comes only after Nothofagus forest is well established. Dung samples of Mylodon confirm that it primarily grazed in open landscapes (40) (e.g., specializing on grasses), suggesting its loss would have little impact on woody vegetation (23).
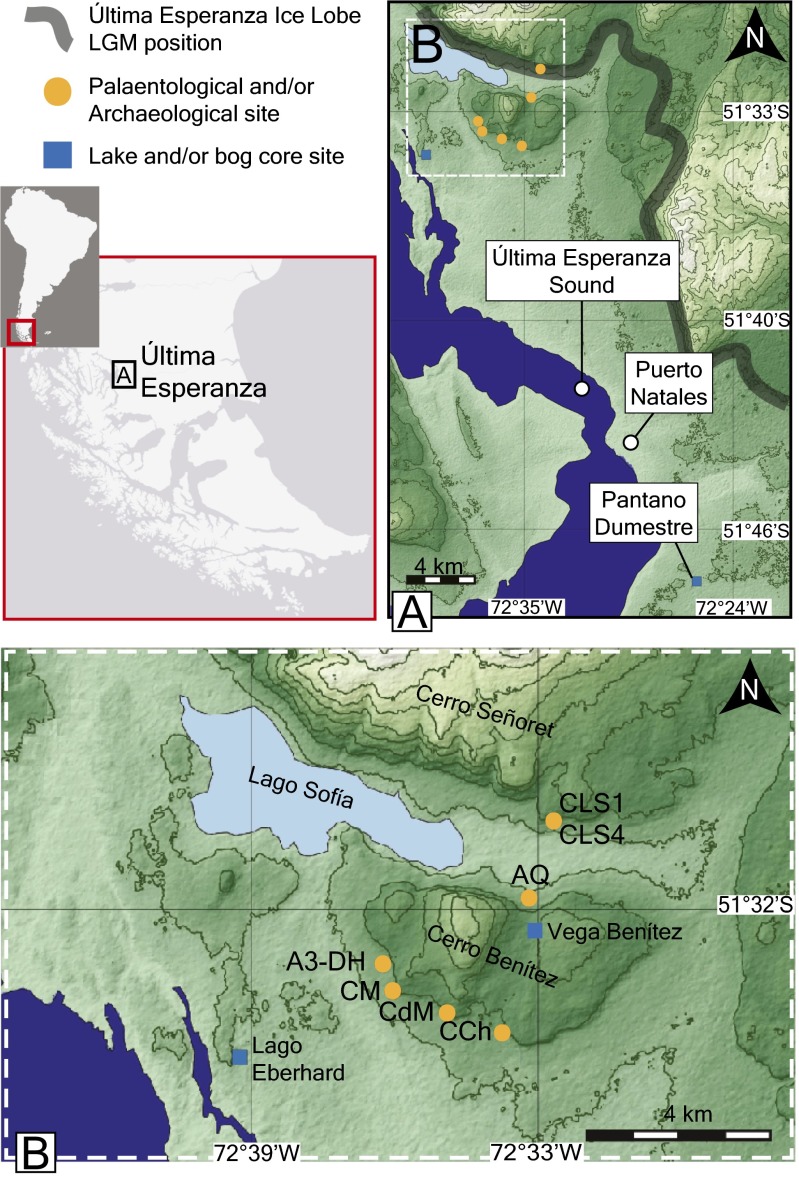
Fig. S1.
(A) General view of the Última Esperanza area. The location of the bog and lake records that provide vegetation change and fire history are shown by the blue rectangles. The megafaunal sites are shown by orange dots. The thick gray line shows the approximate maximum late glacial extent of the Última Esperanza Ice Lobe. Until about 18,000 y ago, a proglacial lake submerged the study area; thus, in this region, it is possible to obtain information about both the earliest records of occupation by megafauna and humans and extinction of the megafauna. (B) Closer view of the Cerro Benítez, Cerro Señoret, and Lago Sofía areas. This is the region that supplies the data discussed in the text and summarized in Fig. S4, which allows building the detailed chronology of human occupation, vegetation change, fire history, and megafaunal extinction that is used to interpret effects of defaunation. A3-DH, Alero 3 Dos Herraduras; AQ, Alero Quemado; CCh, Cueva Chica; CDM, Cueva del Medio; CLS1, Cueva Lago Sofía 1; CLS4, Cueva Lago Sofía 4; CM, Cueva del Milodón. Reproduced with permission from ref. 23.
Fig. S4.
Chronology of megafaunal extinctions plotted against climate change as indicated by oxygen isotope and pollen data (left colored bands, where blue is cool and red is warm); vegetation change [right colored bands, with yellow equating to cold grassland dominance, yellowish green representing mixed grasslands and Nothofagus forest, and olive green (younger than ∼11,000 ka) indicating predominance of Nothofagus forest]; and timing of increase in fire frequency (dotted-line box). See Figs. S2 and S3 for more details on vegetation change and fire frequency indicators. The megafaunal extinction chronology and the chronology of human arrival is based on dates listed in ref. 21; all dates were vetted for robustness, with only those of rank 11 (as explained in ref. 21) or higher used. The timing of local ice-free conditions is taken from ref. 72 (light blue rectangle). Also shown is the timing of the Reclús Volcano eruption (red line), which although it distributed considerable ash in the region, had no apparent effect on the timing of the faunal or vegetation changes (23). The time axis is in calibrated years before present. For the radiocarbon dates on megafauna and human occupation, the dots (or triangles for humans) show the median and the whiskers show the 2-σ probability. Distributions indicating the probability of timing of extinction for megafauna and first arrival for humans were calculated using the GRIWM best-estimate method; see ref. 68 for explanation and R-code. Dietary preferences are indicated in brackets by C (carnivore), M (mixed feeder herbivore), G (grazer), and U (unknown) (40, 73–75). Reproduced with permission from ref. 23.
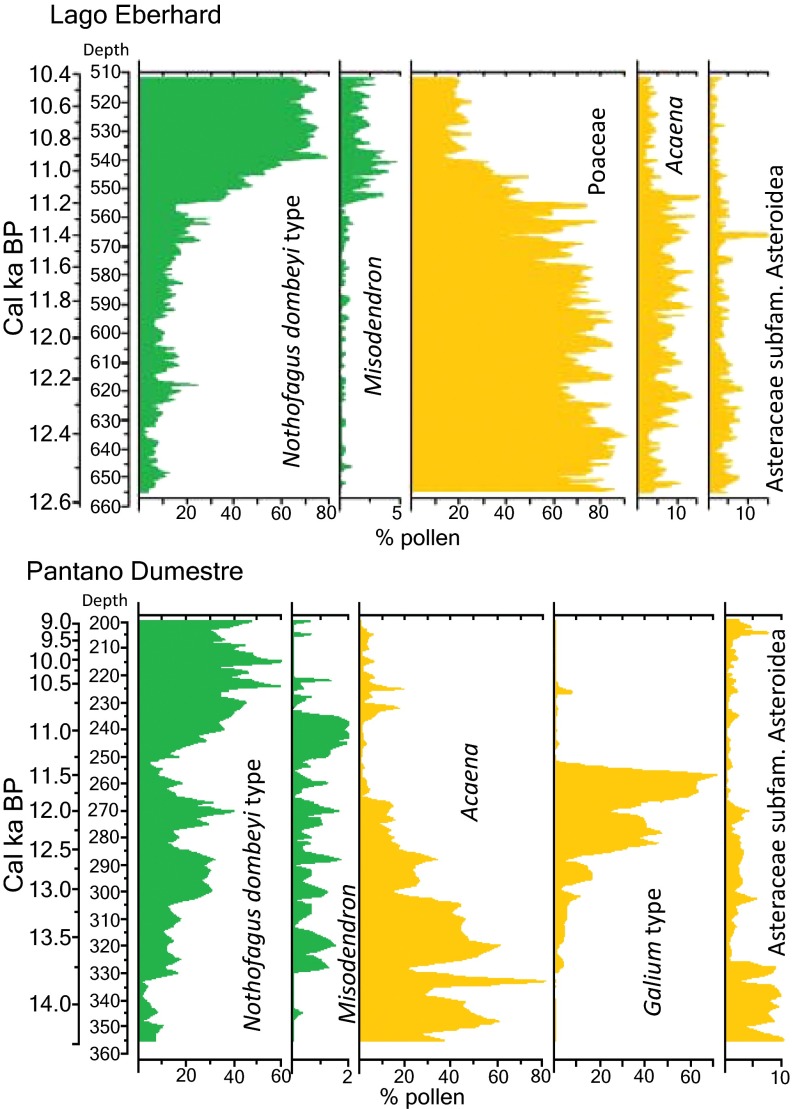
Fig. S2.
Percentage diagram of pollen from Lago Eberhard (Top) and Pantano Dumestre (Bottom). Pollen percentages are shown as the proportion of terrestrial pollen total sum. Only taxa most informative for inferring vegetation changes relevant to megafauna extinction are shown. For a complete presentation and analysis of these records, see refs. 23 and 39. Reproduced with permission from ref. 23.
South America—Pampas.
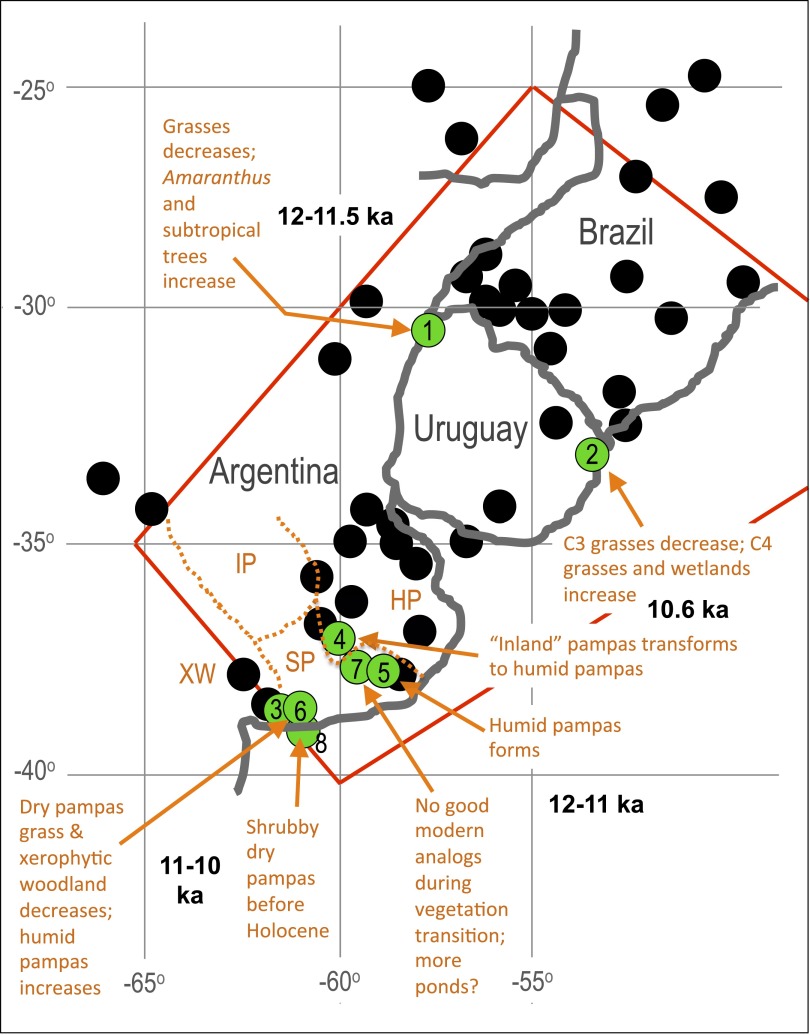
The Pampas region (here considered bounded by 40° S, 60′ W; 35° S, 65′ W; 25° S, 55′ W; 28° S, 45′ W; following ref. 41) (Fig. S5) is located between tropical Brazil and the cold dry Southern Cone. The region contains ecotones that shifted as late-Quaternary climate changed (42) such that the timing of the Pleistocene-to-Holocene vegetation transitions varied across the study area over ∼2,000 y, beginning about 12,000 y ago (Fig. 2) (43–45). Although different parts of the region featured locally distinctive vegetation regimes (see Supporting Information for more detail), in general, the end-Pleistocene Pampas was a C3-dominated grassland steppe. In the Holocene, C4 vegetation increased, as did seasonal variation in proportions of C3 versus C4 grasses (43–45). Overall, the increase in C4 grasses (46) and other vegetational changes (Supporting Information) are consistent with late-Quaternary climatic warming driving a transition to warmer, more humid conditions, as also indicated by sedimentology (47), malacological remains (48), and paleobotanical data (43–45, 49).
Fig. S5.
Red lines delineate the area of the Pampas considered in this paper, which is the area of southeastern South America that comprised a grassland-steppe ecosystem during the Last Glacial Maximum (41). Green dots show the provenance of paleoclimate proxy data that indicate a shift in vegetation and climate in various regions, which took place at somewhat different times over ∼2,000 y beginning ∼12,000 y ago, as explained in the Pampas Region section. Details of the environmental interpretation at each site are given in the following references: ref. 45 (site 1, Pay Paso); ref. 44 (site 2, Los Ajos); ref. 49 (site 3, Arroyo Sauce Chico; site 4, Empalme Querandies; site 5, Cerro La China); and ref. 43 (site 6, Sauce Grandé; site 7, La Horqueta II; site 8, Napostá Grande). Black dots show sites that have yielded Notiomastodon (52), although very few of these specimens have been radiocarbon-dated. Abbreviations for environmental conditions in the late Pleistocene are as follows: HP, Humid Pampas; IP, Inland Pampas; SP, Southern Pampas; XW, xerophytic woodland.
Fig. 2.
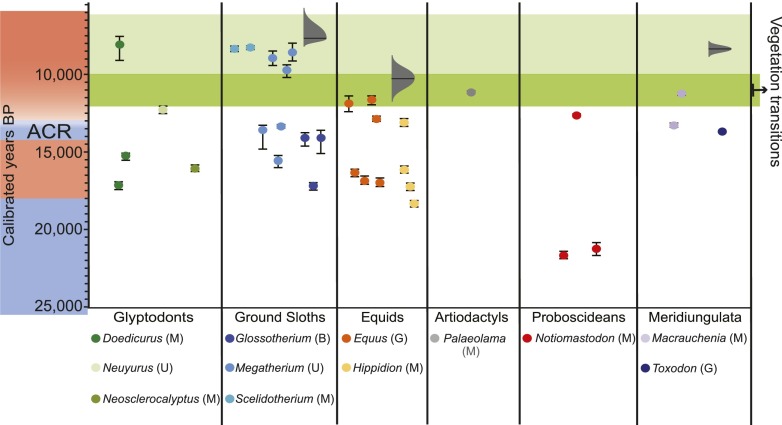
Chronology of megafaunal loss, vegetation, and climate change in the Pampas. The diagram shows robust calibrated radiocarbon dates on bone collagen or tooth enamel (the error bars span the 2-σ probability distribution and, for many dates, are too small to appear on the chart). A transition (darker green shading) from an ecosystem dominated by C3 (Pooid) grasses to C4 (Panicoid) grasses begins ∼12,000 y ago and is asynchronous across the Pampas (see Supporting Information for details). The red and blue shading on the left illustrates cool (blue) and warm (red) times. ACR, Antarctic Cold Reversal, which overlaps the northern hemisphere’s Younger Dryas to some extent but is somewhat earlier. Gray distributions indicating the probability of timing of extinction for megafauna were calculated using the GRIWM best-estimate method (59); also see ref. 68. for R-code. GRIWM’s best estimate of the extinction time and its 95% confidence band are younger than 5,000 y ago for glyptodonts (estimated probable timing of extinction: 1,903 calibrated years BP; range: 4,126 to −256 calibrated years BP; the negative number indicates the probability extends into the future) and Notiomastodon (estimated probable time of extinction: 2,415 calibrated years BP; range: 3,001–1,838 calibrated years BP). Clearly, these anomalously young extinction estimates come from a paucity of radiocarbon dates, with those few available being widely spaced in time. Such results do not provide evidence for persistence of megafauna well into the Holocene; rather, they emphasize that more radiocarbon dates are needed. Dietary preferences of extinct megafauna are indicated by G (grazer), B (browser), M (mixed feeder), and U (unknown) (50, 69).
Most likely well after the vegetation changed (Fig. 2), proboscideans (Notiomastodon), horses (Equus and Hippidion), a llama (Palaeolama), and meridiungulates (Toxodon and Macrauchenia) disappear from the record. Xenarthrans (ground sloths and glyptodonts) go extinct even later (Fig. 2). Based on morphological analyses and isotopic data from the Pampas region, Notiomastodon and Macrauchenia were mixed feeders that consumed both C3 shrubs and C4 grasses; Equus and Toxodon were grazers, and Hippidion consumed primarily C3 vegetation (50). Generally, mammalian herbivores prefer C3 grasses when they are available (46), and in Uruguay today, grazing promotes the dominance of C4 grasses where both C3 and C4 grasses are present, although the effect is variable; C3 and C4 grasses both are heavily used but in different seasons (51). These considerations—in addition to the fact that the grazers and the xenarthrans likely became extinct only well after the vegetation transition began (Fig. 2)—suggest that climate change, not defaunation, was the primary cause of the C3 to C4 transition.
There is no evidence that loss of Notiomastodon significantly impacted the amount of shrubby vegetation in the dry southern pampas (Fig. S5), which actually retracted in range across the Pleistocene–Holocene transition (43, 48). However, many undated Notiomastodon specimens (52) come from northern Uruguay and southern Brazil (Fig. S5), where locally gallery forests along rivers and tropical woodlands expanded somewhat at the end of the Pleistocene (44, 45). More Notiomastodon dates from these areas are needed to assess whether a defaunation signal would emerge. Available data are insufficient to evaluate fire history in this region.
North America—Northeastern United States.
Previous work links megafaunal population crashes with vegetation response and increased fire at Appleman Lake, IN (17, 29); Silver Lake, OH (25); and in southeastern New York (Otisville, Binnewater Pond, Pawelski Farm) (36), using percentage decline of the dung-spore fungus Sporormiella (17, 25, 29, 53, 54) in palynological records as a proxy for local decline of megafauna, with recognition that the taphonomy of Sporormiella is not yet fully understood (24, 53, 55). Vegetation and fire response is consistent with climate change interacting with defaunation (17, 29), but less so with climate change on its own (17, 25, 29). Of interest is the increase in taxa such as Fraxinus nigra (ash), Ostrya/Carpinus (hornbeam/ironwood), and Quercus (oak) in the Indiana and Ohio sites, and Alnus (alder), Betula (birch), and Quercus in the New York sites during the Younger Dryas, a temporary return to cool conditions that presumably would not have favored their expansion. At the same time, charcoal frequencies rose. Increasing percentages of ash, hornbeam/ironwood, oak, alder, and birch and a concomitant increase in fire fits the model of defaunation well, because contemporary ecosystems exhibit such increases in woody understory and tree-forming plants when megafauna, especially elephants, are removed (3, 11, 56, 57), and the increase in combustible woody fuel promotes fires (15–17, 36). The proboscideans Mammut americanum (mastodons) and Mammuthus (mammoths) occupied the region, as did Cervalces (stag-moose), Megalonyx (ground sloth), Ovibos moschatus (shrub ox), Castoroides ohioensis (giant beaver), Platygonus compressus (peccary), and Bison priscus (bison) (58). These megafauna remained on the landscape for >2,000 y after the Sporormiella decline, which occurred by 13.7 ka at Appleman Lake, 13.9 ka at Silver Lake, and between ∼13–14 ka at the New York sites. Summed probability distributions of radiocarbon dates from extinct megafauna in the northeast compiled by ref. 58. suggest significant decline in their abundance by 12,600 y ago, with last records by 11,700 y ago. Confidence intervals calculated by the Gaussian-resampled, inverse-weighted McInerny et al. (GRIWM) method (59) from the data in ref. 58. indicate high probability of extinction by ∼11,000 y ago. Thus, if the Sporormiella decline does indicate significant decrease in megafauna abundance, this decline also indicates that noticeable impacts of defaunation can occur from reducing animal densities even in the absence of final extinction. Also near 11 ka, Quercus pollen (oak) and charcoal abundance increase dramatically, an expected consequence of both megafaunal extinction and warming climate.
North America—Northwestern United States.
We used composite data for the biogeographic region covering northern California into the Pacific Northwest. We correlated palynological and charcoal records from Twin Lake (34) in California’s Klamath Mountains and Mumbo Lake in the Trinity Mountains (35) with mammal data from nearby Potter Creek (60) and Samwell Caves (32, 61), within 100 km to the southeast. Regional timing of extinction is constrained by megafauna dates from Oregon’s Willamette Valley (62) plus the Manis Mastodon (63), all within ∼800 km north of the California cave sites, and Sporormiella declines documented from lake sediments in California’s central Sierra Nevada, about 800 km to the south of the caves (53, 54). Corresponding pollen profiles from the Sporormiella sites have not been published. For proxies of regional vegetation and sea surface temperature, we used information from Ocean Drilling Program (ODP) site 1019 (64).
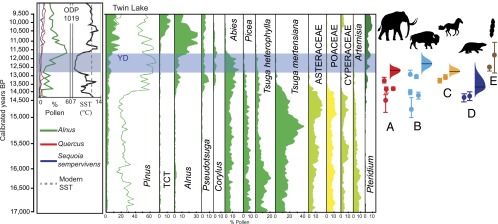
These data document that by 14,000 y ago, megafauna near Twin Lake and Mumbo Lake included Mammuthus (mammoth) and Mammut (mastodon), Bison (bison), Platygonus (peccary), Equus (horse), and the sloths Megalonyx, Paramylodon, and Nothrotheriops (32, 60, 61). The youngest radiocarbon dates and associated confidence intervals indicate that these taxa were likely extinct by ∼12,400 y ago (Fig. 3). Unlike the northeastern United States, where Sporormiella declines long before final extinction of megafauna, in the northwestern United States, the youngest radiocarbon dates on bones and the Sporormiella decline appear generally coeval (Fig. 3).
Fig. 3.
Chronology of vegetation change in northern California compared with sea surface temperature (SST) and regional extinction of megafauna. (Left) SST and pollen-percentage curves are from ODP site 1019 (64). (Center) Twin Lake pollen diagram reproduced with permission from ref. 34. TCT, undifferentiated pollen from Taxodiaceae, Cupressaceae, and Taxaceae. Blue shading indicates Younger Dryas (YD). Megafauna dates are from ref. 62, except for the oldest proboscidean date from ref. 63. (Right) Proboscideans (Mammuthus and Mammut) (A), Bison (B); Equus (C), Paramylodon (D), and Sporormiella (E) from Mono Lake on the left and Exchequer Meadow on the right (53, 54). Probability distributions for extinction timing at right estimated using GRIWM method (59); see Fig. 2 for explanation. The dating of the Sporormiella decline is not amenable to estimating extinction timing with the GRIWM method because of the nature of the data, so here we simply report the 95% uncertainty in the dates used to constrain the ages of the youngest occurrences at each of two separate lake localities.
The Twin Lake record documents that Alnus (alder), a tree-forming shrub that spreads rapidly in the absence of browsing and trampling by ungulates (57), arrived in the region about 14,000 y ago, as the climate became favorable for its growth and when megafauna were still present. Alnus abundance declines slightly at the beginning of the Younger Dryas (∼12,900 y ago), consistent with the expected response to cooler conditions, but then begins to increase in the midst of the Younger Dryas cooling (∼12,000 y ago), at odds with expectations of an exclusively climate-driven change but consistent with predicted response to defaunation (Fig. 3). This is in marked contrast to the persistence of other plant taxa that reflect cool conditions all of the way to the end of the Younger Dryas: Abies (fir), Picea (spruce), and Tsuga heterophylla (western hemlock); these disappear at ∼11,600 y ago. At the same time (from about 12.6–11.8 ka), charcoal abundances indicate that fire frequency increased (34), consistent with expectations of decreased large-herbivore populations.
At Mumbo Lake, both Alnus and Quercus (oaks) were present by ∼13,500 y ago. Beginning ∼12,000 y ago, an increase in evergreen and deciduous oaks and in Alnus, and an inferred transition to a more closed, diverse forest with more burnable biomass, fits expectations of megafaunal loss, although previously those changes have been attributed entirely to climatic drivers (35).
Climate proxy and vegetation data from ODP site 1019 suggest that regionally vegetation changed in the mid-Younger Dryas in ways that are inconsistent with expectations from climate change alone (64). Especially suggestive of defaunation is the dramatic rise in Alnus pollen, which precedes, by about 600 y, the warming of local sea surface temperatures (Fig. 3).
North America—Alaska and the Yukon (Beringia).
Past work has pointed out that end-Pleistocene vegetation changes in Beringia are consistent with defaunation (26, 27, 33) (summarized in Supporting Information). The ecological transformation from productive forests and grasslands to tundra has been interpreted (26, 27) to result from the loss of mammoths plus human-caused reduction in the densities of megafaunal species that survived. With fewer megafauna on the landscape and proboscideans absent, the reduction of trampling, browsing, and grazing pressure by big animals is postulated to have stimulated an increase in woody and leafy plants, which set off an ecological cascade by promoting accumulation of surface leaf litter that insulated soil, reduced summer soil temperatures, caused formation of permafrost, and favored growth of mosses and shrubs at the expense of grasses and other nutritious fodder for herbivores. Experimental studies support this idea (26, 27, 65).
Mammal Communities
Of regions discussed in this paper, only northern California has an adequate published record, from Samwell Cave (32), to test for local impacts of defaunation on mammalian community structure. Samwell Cave is near the two pollen sites discussed in North America—Northwestern United States. There, coeval with the regional signal for megafaunal extinction, a lasting decline in both richness and evenness (a metric describing to what degree abundances of individuals in different taxa are equal) in the small mammal community has been well documented (32). At the continental scale and within biogeographic provinces, most mammalian communities in the lower 48 United States also became less species-rich during the time when megafauna extinction was accelerating (30, 31) (also see Supporting Information). Such diversity losses at the local to continental scales are consistent with megafaunal extinction triggering adjustments of small-mammal density and distribution.
Discussion
Pinpointing a particular taxon’s extinction precisely is not possible given vagaries of the fossil record; therefore, we have expressed extinction timing in terms of confidence bands (Figs. 2 and 3 and Fig. S4; also see ref. 23). Wide bands result from a paucity of dates, a limiting factor in our approach. Also, the fossil record is likely to preserve only the most dramatic, obvious signs of defaunation. This is both a strength and a weakness of our study. The strength is that if the fossil record does reveal effects of losing megafauna, those effects are severe and the implication that they have for ongoing defaunation are robust. The weakness is that subtler but perhaps equally important effects of defaunation, such as changes in nutrient cycling or in environmental heterogeneity, may not be revealed by our methods. With those caveats, the data available warrant some tentative conclusions.
In all regions examined, end-Pleistocene climate change clearly played a role in reshaping ecosystems, as is widely recognized (17–19, 21, 23, 25–27, 29–35). However, in all three North American regions, a common aftermath of losing herbivore megafauna was an apparent increase in plants that form understory and deciduous forest; this was not observed for either of the South American regions. These differences may illustrate an important point about potential effects of megafauna loss—the extent of ecological change depends on: (i) the ecological roles of the deleted megafauna; and (ii) the chance abiotic constraints—including climate and historical contingency—that provide opportunities for marked transformation of vegetation. In the North American sites, a critical ecosystem engineer seems to have been proboscideans; only after mammoths and/or mastodons disappeared or declined substantially did denser understory and deciduous forests began to flourish, much as is evident in African savannah-grasslands today (Fig. 4).
Fig. 4.
Amboseli National Park, Kenya. Typical vegetation is shown where elephants roam freely (A) compared with a nearby area where elephants are excluded (B).
In southern Patagonia, forest-forming vegetation—Nothofagus—was there, but proboscideans were not. This finding may explain why, in this study area, the signal for defaunation is at best equivocal for extinction of an equid and a llama and absent for deletion of the mylodont sloth. The lost taxa were not ecosystem engineers in the sense of knocking over trees, tearing off their branches, and trampling and eating new shoots. Instead, the lost taxa inhabited open grasslands almost exclusively; only when the grasslands disappeared, as a result of climatic changes that allowed Nothofagus forest to become widespread, did those megafauna disappear from the region.
In the Pampas a forest-ecosystem engineer, Notiomastodon, was present (and lost), but in most of the region that contains dated Notiomastodon fossils, either the soil and climatic regimes remained unsuitable for the establishment of forests, or tree species capable of thriving in that environment never dispersed there, so even after Notiomastodon disappeared, major restructuring of the vegetational component of the ecosystem did not result. Further work is needed to see whether loss of Notiomastodon might help explain local early-Holocene expansion of forests in the northern, more tropical pampas (northernmost Uruguay and southeastern Brazil).
Conclusions
Our data provide a test of whether many different kinds of megafauna—such as proboscideans, giant ground sloths, glyptodonts, toxodonts, equids, and llamas—cause similar, lasting ecological changes when they go extinct. We find that the clear and lasting defaunation effects are variable, depending on the megafauna species and the particular ecosystem from which it was deleted. In general, it seems extinction of megafauna will likely trigger permanent ecological state shifts that manifest as substantially modified vegetation and mammalian community structure if (i) the lost megafauna are significant ecosystem engineers; and (ii) the ecosystem contains plant species susceptible to ecological release with declining herbivore pressure. The North American systems we studied, which contained proboscideans, showed particularly strong response to defaunation. The two South American areas we studied did not exhibit as strong a defaunation signal, either because megafauna that acted as major forest-ecosystem engineers were absent (southwestern Patagonia, which lacked proboscideans) or because soil and climatic limitations prevented the plant taxa capable of forming dense forest to flourish, or the requisite plants never dispersed there (the Pampas).
As has been previously recognized (17, 25, 29), the ecosystems that are most prone to shift into new regimes are those that are simultaneously impacted by both climate change, which sets the stage for previously minor components of vegetation to flourish, and defaunation, which further promotes major vegetational transformations. Both conditions are present in contemporary ecosystems with megafauna, as they were during the Pleistocene–Holocene transition.
Our study also provides insights about which modern-day ecosystems are most in danger of disappearing as a result of contemporary defaunation. Risks of irreversible change may be highest in mixed mosaics of grassland and forest that still contain the largest megafauna, which tend to be ecosystem engineers. For example, large swaths of savannah in East and South Africa, where elephants are in danger of extinction within the next two decades from intense poaching, would be likely to transform radically (Fig. 4). However, because our study demonstrates that not all megafaunal species play equal roles in maintaining ecosystem structure and function, it highlights the necessity to thoroughly understand the ecological role of each species before making predictions about how its removal will or will not cascade through an ecosystem to trigger irreversible changes.
Methods
Following refs. 21 and 66, we assume that the actual extinction of a given species was more recent than its youngest dated specimens, so we calculated estimates of the temporal intervals over which taxa became regionally extinct using the GRIWM method (59) (Figs. 2 and 3). GRIWM provides an estimate of the true time of extinction by extending the observed stratigraphic range by the average gap size (but up-weighting younger gaps to accommodate nonrandom preservation and recovery of fossils) within the known stratigraphic range. GRIWM accommodates the uncertainties in the radiometric dates by providing a 95% confidence band around the estimated extinction time. All dates mentioned in the text were vetted from published literature and are expressed in calendar years before present calibrated using Calib version 7.0.2 (67). For South American megafauna dates, only radiocarbon dates considered robust (with a score of ≥11, following ref. 21) were used.
Última Esperanza Site, Southwestern Patagonia
Details of the Última Esperanza analysis have been published elsewhere (23). Here, we provide summary figures and information most pertinent for interpreting the impacts of defaunation.
Fig. S1 shows the location of the megafaunal and pollen sites in the study area, which is in southern Chile near the eastern flank of the Andes Mountains, within a climatic regime that today supports a mixed Nothofagus forest. Note that the megafauna sites and the lakes that provide paleoenvironmental information (vegetation, climate, and fire histories) are all found within about 30 km of each other, most within 10 km, making this a highly resolved record of events in a small geographic region.
Figs. S2 and S3 show relevant data on which the interpretation of the transition to Nothofagus forest from the preexisting cool grasslands is based, and charcoal records from which fire history is inferred.
Fig. S3.
Comparison of Lago Eberhard (Left) and Pantano Dumestre (Right) charcoal and vegetation-change records. Traces A and B indicate percentage of organic matter through time; traces C and D indicate percentage of grass charcoal; traces E and F indicate fire frequency, measured as fire events/500 y; traces G and H indicate Charcoal Accumulation Rates analyses (CHAR); traces I and J indicate rates of change analyses (ROC) in the pollen records. Information is extracted and modified from refs. 23 and 39. Black triangles show the timing of the onset in the increase of Nothofagus for each site. Reproduced with permission from ref. 23.
Fig. S4 summarizes the available radiocarbon dates for all megafauna found from the sites shown in Fig. S1, compared with a summary of climate change, fire frequency, and human arrival. As explained in ref. 23, the GRIWM best-estimates of the time of extinction (or arrival in the case of the humans) is indicated by the colored normal distributions, which represent the 95% confidence intervals (black line in Fig. S4, most probable time of extinction). Warming and cooling events are inferred from the Epica Dome C Antarctic ice core (70) and are consistent with the pollen chronologies; red and blue bands at left in Fig. S4 summarize climate data as warm versus cooling events, respectively. Note that the ACR is a temporary return to cold conditions, which occurred after end-Pleistocene warming had commenced and had caused glaciers to retreat from their maximum extent. The ACR occurs slightly earlier than, but overlaps, the Younger Dryas in North America. Major vegetation changes (yellow, light green, and dark green bands at right in Fig. S4) and fire frequency information (black dashed rectangle in Fig. S4) are extracted from ref. 39; see also Figs. S2 and S3.
Pampas Region
Fig. S5 shows boundaries of the Pampas as considered in this study, the location of paleoenvironmental proxy sites, and the location of specimens of Notiomastodon, both dated and undated. The data presented in Fig. 2 of the main text were compiled from recent literature, as cited in the main text. Radiocarbon dates for megafauna were taken primarily from refs. 21 and 22 and vetted so that only dates with rank 11 or above (as explained in ref. 21) were used. The paleovegetation and paleoclimatic proxies as interpreted by the studies cited in Fig. S5 were used to compile the vegetation-change and climatic histories of the region. Although the general vegetation change across the entire study area involved a shift from C3-to-C4 grasslands, regionally across the Pampas distinctive patterns of change are apparent (Fig. S5): (i) grassland to Amaranthus steppe and expansion of subtropical forest in the north-central pampas between 12,000–11,500 y ago (45); (ii) C3 to C4-dominated grasslands and widespread development of wetland and floodplain vegetation in the easternmost regions between 12,000–10,600 y ago (43, 44); and (iii) replacement of shrub steppe and xerophytic woodland with humid grassland in the southernmost Pampas between ∼11,000–10,000 y ago (43).
North America—Beringia (Alaska and the Yukon)
Refs. 26, 27, and 33 present detailed information to track the changing abundance and extinction of megafauna with respect to vegetation changes. The basic chronology of relevance to assessing vegetational impacts of megafaunal loss is that Equus drops out of the record first, by ∼12,300 y ago, coincident with arrival of humans and with increasing numbers of Cervus elaphus (wapiti) (26, 27, 33). Contemporaneous vegetation changes—increase in Salix (willow), Gramineae (grasses), Cyperaceae (sedges), Artemisia (sagebrush), and Betula (dwarf birch)—are more parsimoniously explained by climate change to warmer, moister conditions than by replacing horses with wapiti. Mammoths remained until ∼11,200 y ago; with their decrease (27), Betula increased rapidly, leveling off at peak abundance coincident with the youngest radiocarbon dates on mammoth bones. The pattern of increasing birch forests with loss of mammoths is consistent with removing proboscideans from the ecosystem (26, 27, 33). Coeval with last records of mammoths were apparently decreasing abundances of wapiti and bison, along with an increase in human population densities, further climatic warming, and conversion of vast tracts of formerly productive forests and grasslands to tundra.
Mammal Communities
The richness decline of mammal communities at the continental scale had two components, actual extinction of megafauna, and individualistic range adjustments of the small-bodied mammal species. The small-bodied species did not suffer much extinction, with only 6 species known to die out, in contrast to at least 60 large-bodied ones. The biogeographic adjustments of small mammals, however, apparently contributed considerably to depressing richness: causing small mammal diversity to decline by 16–51% (depending on the biogeographic province), effectively doubling the regional diversity loss attributable to megafaunal extinction alone (30).
Detailed diversity studies are not available for the South American regions addressed in this paper, but low richness and evenness has been noted in early-Holocene rodent communities in the Pampas (71).
Acknowledgments
We thank Y. Mahli and C. Doughty for inviting us to present this work. F. Martín and L. Borrero helped in assembling data from southern Patagonia. J. L. Prado provided a preprint of ref. 22. We appreciate discussions with R. Byrne, G. Politis, J. L. Prado, L. Avilla, and D. Mothé. This work was funded by National Science Foundation Earth Sciences Grant 1148181. This is University of California Museum of Paleontology Contribution 2072.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
2Present address: Instituto de Ciencias de la Tierra, Facultad de Ciencias, Universidad Austral de Chile, Valdivia, Chile.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1505295112/-/DCSupplemental.
References
- 1.Dirzo R, et al. Defaunation in the Anthropocene. Science. 2014;345(6195):401–406. doi: 10.1126/science.1251817. [DOI] [PubMed] [Google Scholar]
- 2.McCauley DJ, et al. Marine defaunation: Animal loss in the global ocean. Science. 2015;347(6219):1255641. doi: 10.1126/science.1255641. [DOI] [PubMed] [Google Scholar]
- 3.Estes JA, et al. Trophic downgrading of planet Earth. Science. 2011;333(6040):301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- 4.Young HS, et al. Declines in large wildlife increase landscape-level prevalence of rodent-borne disease in Africa. Proc Natl Acad Sci USA. 2014;111(19):7036–7041. doi: 10.1073/pnas.1404958111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young HS, et al. Effects of mammalian herbivore declines on plant communities: Observations and experiments in an African savanna. J Ecol. 2013;101(4):1030–1041. doi: 10.1111/1365-2745.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corlett RT. The shifted baseline: Prehistoric defaunation in the tropics and its consequences for biodiversity conservation. Biol Conserv. 2013;163:13–21. [Google Scholar]
- 7.Galetti M, Dirzo R. Ecological and evolutionary consequences of living in a defaunated world. Biol Conserv. 2013;163:1–6. [Google Scholar]
- 8.Kay CE. Are ecosystems structured from the top-down or bottom-up: A new look at an old debate. Wildl Soc Bull. 1998;26(3):484–498. [Google Scholar]
- 9.Kurten EL. Cascading effects of contemporaneous defaunation on tropical forest communities. Biol Conserv. 2013;163:22–32. [Google Scholar]
- 10.Reider KE, Carson WP, Donnelly MA. Effects of collared peccary (Pecari tajacu) exclusion on leaf litter amphibians and reptiles in a Neotropical wet forest, Costa Rica. Biol Conserv. 2013;163:90–98. [Google Scholar]
- 11.Owen-Smith N. Pleistocene extinctions: The pivotal role of megaherbivores. Paleobiology. 1987;13:351–362. [Google Scholar]
- 12.Frank DA, McNaughton SJ, Tracy BF. The ecology of the Earth’s grazing ecosystems. Bioscience. 1998;48:513–521. [Google Scholar]
- 13.Doughty CE, Wolf A, Malhi Y. The legacy of the Pleistocene megafauna extinctions on nutrient availability in Amazonia. Nat Geosci. 2013;6:761–764. [Google Scholar]
- 14.Beaune D, Fruth B, Bollache L, Hohmann G, Bretagnolle F. Doom of the elephant-dependent trees in a Congo tropical forest. For Ecol Manage. 2013;295:109–117. [Google Scholar]
- 15.Bond WJ. Large parts of the world are brown or black: A different view on the ‘Green World’ hypothesis. J Veg Sci. 2005;16:261–266. [Google Scholar]
- 16.Bond WJ, Keeley JE. Fire as a global ‘herbivore’: The ecology and evolution of flammable ecosystems. Trends Ecol Evol. 2005;20(7):387–394. doi: 10.1016/j.tree.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 17.Gill JL. Ecological impacts of the late Quaternary megaherbivore extinctions. New Phytol. 2014;201(4):1163–1169. doi: 10.1111/nph.12576. [DOI] [PubMed] [Google Scholar]
- 18.Barnosky AD, et al. Approaching a state shift in Earth’s biosphere. Nature. 2012;486(7401):52–58. doi: 10.1038/nature11018. [DOI] [PubMed] [Google Scholar]
- 19.Koch PL, Barnosky AD. Late Quaternary extinctions: State of the debate. Annu Rev Ecol Evol Syst. 2006;37:215–250. [Google Scholar]
- 20.Wroe S, et al. Climate change frames debate over the extinction of megafauna in Sahul (Pleistocene Australia-New Guinea) Proc Natl Acad Sci USA. 2013;110(22):8777–8781. doi: 10.1073/pnas.1302698110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnosky AD, Lindsey EL. Timing of Quaternary megafaunal extinction in South America in relation to human arrival and climate change. Quat Int. 2010;217:10–29. [Google Scholar]
- 22.Prado JL, Martinez-Maza C, Alberdi MT. Megafauna extinction in South America: A new chronology for the Argentine Pampas. Palaeogeogr Palaeoclimatol Palaeoecol. 2015;425:41–49. [Google Scholar]
- 23.Villavicencio N, et al. Combination of humans, climate, and vegetation change triggered Late Quaternary megafauna extinction in the Última Esperanza region, southern Patagonia, Chile. Ecography. 2015 doi: 10.1111/ecog.01606. [DOI] [Google Scholar]
- 24.Gill JL, McLauchlan KK, Skibbe AM, Goring SJ, Williams JW. Linking abundances of the dung fungus Sporormiella to the density of bison: Implications for assessing grazing by megaherbivores in palaeorecords. J Ecol. 2013;101(5):1125–1136. [Google Scholar]
- 25.Gill JL, Williams JW, Jackson ST, Donnelly JP, Schellinger GC. Climatic and megaherbivory controls on late-glacial vegetation dynamics: A new, high-resolution, multi-proxy record from Silver Lake, Ohio. Quat Sci Rev. 2012;34:68–80. [Google Scholar]
- 26.Zimov SA, et al. Steppe-tundra transition: A herbivore-driven biome shift at the end of the pleistocene. Am Nat. 1995;146(5):765–794. [Google Scholar]
- 27.Zimov SA, Zimov NS, Chapin FS., III . The past and future of the mammoth steppe ecosystem. In: Louys J, editor. Paleontology in Ecology and Conservation. Springer; Heidelberg: 2012. pp. 193–226. [Google Scholar]
- 28.Rule S, et al. The aftermath of megafaunal extinction: Ecosystem transformation in Pleistocene Australia. Science. 2012;335(6075):1483–1486. doi: 10.1126/science.1214261. [DOI] [PubMed] [Google Scholar]
- 29.Gill JL, Williams JW, Jackson ST, Lininger KB, Robinson GS. Pleistocene megafaunal collapse, novel plant communities, and enhanced fire regimes in North America. Science. 2009;326(5956):1100–1103. doi: 10.1126/science.1179504. [DOI] [PubMed] [Google Scholar]
- 30.Barnosky AD, Carrasco MA, Graham RW. Collateral mammal diversity loss associated with late Quaternary megafaunal extinctions and implications for the future, Geological Society Special Publications 358. In: McGowan AJ, Smith AB, editors. Comparing the Geological and Fossil Records: Implications for Biodiversity Studies. Geological Society; London: 2011. pp. 179–189. [Google Scholar]
- 31.Graham RW, et al. Spatial response of mammals to late Quaternary environmental fluctuations. Science. 1996;272(5268):1601–1606. doi: 10.1126/science.272.5268.1601. [DOI] [PubMed] [Google Scholar]
- 32.Blois JL, McGuire JL, Hadly EA. Small mammal diversity loss in response to late-Pleistocene climatic change. Nature. 2010;465(7299):771–774. doi: 10.1038/nature09077. [DOI] [PubMed] [Google Scholar]
- 33.Guthrie RD. New carbon dates link climatic change with human colonization and Pleistocene extinctions. Nature. 2006;441(7090):207–209. doi: 10.1038/nature04604. [DOI] [PubMed] [Google Scholar]
- 34.Wanket JA. 2002. Late Quaternary vegetation and climate of the Klamath Mountains. PhD dissertation (Univ of California, Berkeley)
- 35.Daniels ML, Anderson RS, Whitlock C. Vegetation and fire history since the Late Pleistocene from the Trinity Mountains, northwestern California, USA. Holocene. 2005;15(7):1062–1071. [Google Scholar]
- 36.Robinson GS, Burney LP, Burney DA. Landscape paleoecology and megafaunal extinction in southeastern New York State. Ecol Monogr. 2005;75(3):295–315. [Google Scholar]
- 37.Dirzo R, Miranda A. Contemporary neotropical defaunation and forest structure, function, and diversity - A sequel to John Terborgh. Conserv Biol. 1990;4:444–447. [Google Scholar]
- 38.Weinstock J, et al. The Late Pleistocene distribution of vicuñas (Vicugna vicugna) and the “extinction” of the gracile llama (“Lama gracilis”): New molecular data. Quat Sci Rev. 2009;28(15):1369–1373. [Google Scholar]
- 39.Moreno PI, Villa-Martínez R, Cárdenas ML, Sagredodo EA. Deglacial changes of the southern margin of the southern westerly winds revealed by terrestrial records from SW Patagonia (52° S) Quat Sci Rev. 2012;41:1–21. [Google Scholar]
- 40.Markgraf V. Late pleistocene faunal extinctions in southern patagonia. Science. 1985;228(4703):1110–1112. doi: 10.1126/science.228.4703.1110. [DOI] [PubMed] [Google Scholar]
- 41.Cione AL, Tonni EP, Soibelzon L. Did humans cause the late Pleistocene-early Holocene mammalian extinctions in South America in a context of shrinking open areas? In: Haynes G, editor. American Megafaunal Extinctions at the End of the Pleistocene. Springer; New York: 2009. pp. 125–144. [Google Scholar]
- 42.Prado JL, Alberdi MT. Quaternary mammalian faunas of the Pampean Region. Quat Int. 2010;212:176–186. [Google Scholar]
- 43.Prieto AR. Vegetational history of the Late glacial–Holocene transition in the grasslands of eastern Argentina. Palaeogeogr Palaeoclimatol Palaeoecol. 2000;157:167–188. [Google Scholar]
- 44.Iriarte J. Vegetation and climate change since 14,810 14C yr B.P. in southeastern Uruguay and implications for the rise of early Formative societies. Quat Res. 2006;65:20–32. [Google Scholar]
- 45.Suárez R. 2010. Arqueología durante la transición Pleistoceno-Holoceno: Componentes paloindios, organización de la tecnología lítica and movilidad de los primeros americanos en Uruguay. PhD dissertation (Universidad de la Plata, La Plata, Argentina)
- 46.Anderson RC. Evolution and origin of the Central Grassland of North America: Climate, fire, and mammalian grazers. J Torrey Bot Soc. 2006;133(4):626–647. [Google Scholar]
- 47.Quattrocchio ME, Borromeia AM, Deschamps CM, Grilla SC, Zavala CA. Landscape evolution and climate changes in the Late Pleistocene–Holocene, southern Pampa (Argentina): Evidence from palynology, mammals and sedimentology. Quat Int. 2008;181:123–138. [Google Scholar]
- 48.Prieto AR, Blasi AM, Francesco CGD, Fernández C. Environmental history since 11,000 14C yr B.P. of the northeastern Pampas, Argentina, from alluvial sequences of the Luján River. Quat Res. 2004;62:146–161. [Google Scholar]
- 49.Prieto AR. Late Quaternary vegetational and climatic changes in the Pampa grassland of Argentina. Quat Res. 1996;45:73–88. [Google Scholar]
- 50.França LM, et al. Review of feeding ecology data of Late Pleistocene mammalian herbivores from South America and discussions on niche differentiation. Earth Sci Rev. 2015;140:158–165. [Google Scholar]
- 51.Altesor A, Oesterheld M, Leoni E, Lezama F, Rodríguez C. Effect of grazing on community structure and productivity of a Uruguayan grassland. Plant Ecol. 2005;179(1):83–91. [Google Scholar]
- 52.Mothé D, Avilla LS, Cozzuol MA. The south American gomphotheres (Mammalia, Proboscidea, Gomphotheriidae): Taxonomy, phylogeny, and biogeography. J Mamm Evol. 2013;20(1):23–32. [Google Scholar]
- 53.Davis OK, Shafer DS. Sporormiella fungal spores, a palynological means of detecting herbivore density. Palaeogeogr Palaeoclimatol Palaeoecol. 2002;237(1):40–50. [Google Scholar]
- 54.Faith JT. Late Pleistocene climate change, nutrient cycling, and the megafaunal extinctions in North America. Quat Sci Rev. 2011;30(13-14):1675–1680. [Google Scholar]
- 55.Feranec RS, Miller NG, Lothrop JC, Graham RW. The Sporormiella proxy and end-Pleistocene megafaunal extinction: A perspective. Quat Int. 2011;245(2):333–338. [Google Scholar]
- 56.Cumming DHM, et al. Elephants, woodlands and biodiversity in miombo woodland in southern Africa. S Afr J Sci. 1997;93:231–236. [Google Scholar]
- 57.Alldredge MW, Peek JM, Wall WA. Alterations of shrub communities in relation to herbivory in northern Idaho. Northwest Sci. 2001;75(2):137–144. [Google Scholar]
- 58.Boulanger MT, Lyman RL. Northeastern North American Pleistocene megafauna chronologically overlapped minimally with Paleoindians. Quat Sci Rev. 2014;85:35–46. [Google Scholar]
- 59.Bradshaw CJA, Cooper A, Turney CSM, Brook BW. Robust estimates of extinction time in the geological record. Quat Sci Rev. 2012;33:14–19. [Google Scholar]
- 60.Feranec RS. Implications of radiocarbon dates from Potter Creek Cave, Shasta County, California, USA. Radiocarbon. 2009;51(3):931–936. [Google Scholar]
- 61.Feranec RS, Hadly EA, Blois JL, Barnosky AD, Paytan A. Radiocarbon dates from the Pleistocene fossil deposits of Samwel Cave, Shasta County, California, USA. Radiocarbon. 2007;49(1):117–121. [Google Scholar]
- 62.Gilmour DM, et al. Chronology and ecology of late Pleistocene megafauna in the northern Willamette Valley, Oregon. Quat Res. 2015;83:127–136. [Google Scholar]
- 63.Waters MR, et al. Pre-Clovis mastodon hunting 13,800 years ago at the Manis site, Washington. Science. 2011;334(6054):351–353. doi: 10.1126/science.1207663. [DOI] [PubMed] [Google Scholar]
- 64.Barron JA, Heusser L, Herbert T, Lyle M. High-resolution climatic evolution of coastal northern California during the past 16,000 years. Paleoceanography. 2003;18(1):1020. [Google Scholar]
- 65.Olofsson J, Stark S, Oksanen L. Reindeer influence on ecosystem processes in the tundra. Oikos. 2003;105:386–396. [Google Scholar]
- 66.Marshall CR. Confidence intervals on stratigraphic ranges. Paleobiology. 1990;16:1–10. [Google Scholar]
- 67.Stuiver M, Reimer PJ, Reimer R. 2015. CALIB Radiocarbon Calibration. Available at: calib.qub.ac.uk/calib. Accessed August 26, 2015.
- 68.Fé S, et al. Uncertainties in specimen dates constrain the choice of statistical method to infer extinction time. Quat Sci Rev. 2015;112:128–137. [Google Scholar]
- 69.Czerwonogora A, Fariña RA, Tonni EP. Diet and isotopes of Late Pleistocene ground sloths: First results for Lestodon and Glossotherium (Xenarthra, Tardigrada) Neues Jahrb Geol Palaontol Abh. 2011;262(3):257–266. [Google Scholar]
- 70.Jouzel J, et al. Orbital and millennial Antarctic climate variability over the past 800,000 years. Science. 2007;317(5839):793–796. doi: 10.1126/science.1141038. [DOI] [PubMed] [Google Scholar]
- 71.Pardiñas UF. Condiciones áridas durante el Holoceno Temprano en el sudoeste de la provincia de Buenos Aires (Argentina): Vertebrados y tafonomía. Ameghiniana. 2014;38(3):227–236. [Google Scholar]
- 72.Sagredo EA, et al. Fluctuations of the Última Esperanza ice lobe (52°S), Chilean Patagonia, during the last glacial maximum and termination 1. Geomorphology. 2011;125:92–108. [Google Scholar]
- 73.García A, Carretero EM, Dacar MA. Presence of Hippidion at two sites of western Argentina: Diet composition and contribution to the study of the extinction of Pleistocene megafauna. Quat Int. 2008;180:22–29. [Google Scholar]
- 74.Cajal JL. Uso de hábitat por vicuñas y guanacos en la Reserva San Guillermo, Argentina. Vida Silvestre Neotropical. 1989;2:21–31. [Google Scholar]
- 75.Puig S. Uso de los recursos naturales por el guanaco. In: Puig S, editor. Técnicas Para el Manejo del Guanaco, UICN. Unión Internacional para la Conservación de la Naturaleza; Gland, Switzerland: 1995. pp. 110–126. [Google Scholar]