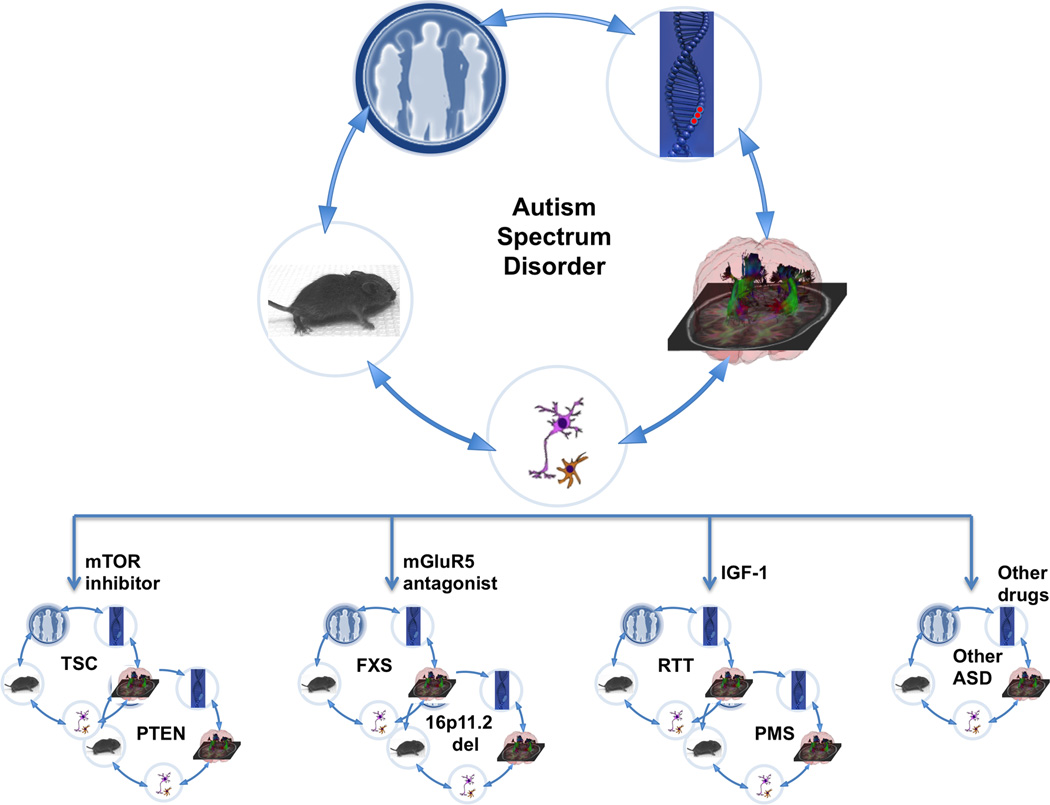
Figure 3. Translational research and clinical trials in ASD.
Translational studies in ASD have gained momentum from genetically defined causes such as FXS, TSC and RTT. The patients with these disorders are phenotyped in detail using advanced imaging and electrophysiology studies, with the aim of identifying potential biomarkers. There are cell-based models (both rodent and human) as well as mouse models of these syndromes enabling preclinical trials. Together, these efforts have led to clinical trials in some of these disorders. Based on the preclinical trials, the hypothesis is that different etiologies of ASD will respond to different therapies such as mTOR inhibitors (TSC and PTEN), mGluR5 antagonists (FXS and 16p11.2 deletion) and IGF-1 (RTT and PMS). Subsets of non-syndromic ASD patients may also benefit from one of these therapies, but further studies will be required to provide the tools and methods to stratify the individuals with non-syndromic ASD into treatment groups. It is important to remember that the discovery cycle will likely take more than one round in order to achieve safe and effective therapies for these disorders.