Abstract
Climate change and decadal variability are impacting marine fish and invertebrate species worldwide and these impacts will continue for the foreseeable future. Quantitative approaches have been developed to examine climate impacts on productivity, abundance, and distribution of various marine fish and invertebrate species. However, it is difficult to apply these approaches to large numbers of species owing to the lack of mechanistic understanding sufficient for quantitative analyses, as well as the lack of scientific infrastructure to support these more detailed studies. Vulnerability assessments provide a framework for evaluating climate impacts over a broad range of species with existing information. These methods combine the exposure of a species to a stressor (climate change and decadal variability) and the sensitivity of species to the stressor. These two components are then combined to estimate an overall vulnerability. Quantitative data are used when available, but qualitative information and expert opinion are used when quantitative data is lacking. Here we conduct a climate vulnerability assessment on 82 fish and invertebrate species in the Northeast U.S. Shelf including exploited, forage, and protected species. We define climate vulnerability as the extent to which abundance or productivity of a species in the region could be impacted by climate change and decadal variability. We find that the overall climate vulnerability is high to very high for approximately half the species assessed; diadromous and benthic invertebrate species exhibit the greatest vulnerability. In addition, the majority of species included in the assessment have a high potential for a change in distribution in response to projected changes in climate. Negative effects of climate change are expected for approximately half of the species assessed, but some species are expected to be positively affected (e.g., increase in productivity or move into the region). These results will inform research and management activities related to understanding and adapting marine fisheries management and conservation to climate change and decadal variability.
Introduction
Marine fish and invertebrate species are impacted by climate change and decadal variability. A classic example is the historical oscillation between Pacific Sardine and Northern Anchovy populations in the California Current, which occurred before recorded human exploitation began [1]. More recently, changes in marine species distribution and population productivity have been linked to changes in the climate [2–5]. Changes have also been documented in the distribution of fishery landings and potentially the distribution and magnitude of fishing effort [6, 7]. Although fishing remains an important, and in many cases, dominant driver of population abundance, there is now substantial evidence that climate change and decadal variability affect fish and invertebrate populations [8–10].
An increasing number of studies are linking population models to climate models and projecting the effect of future climate change on marine fish and invertebrate species [11–16]. These studies develop either a process-based or empirical relationship between climate variables and population parameters. Projections of the climate factor from climate models are then used to force the population model into the future [17]. In general, these studies show that climate change will continue to impact species and the ecosystem services they provide (e.g., fisheries, forage, [18]) for the foreseeable future (decades to centuries). For many regions, developing a mechanistic model for each species is not possible in the short-term because of the limited personnel and scientific resources, the lack of mechanistic models linking climate to population dynamics, and the large number of managed species. Global and regional species distribution models have been linked to climate projections to project changes in available habitat and resulting distribution shifts [19, 20]. These studies typically do not focus on providing species-specific information that can be used by regional fisheries managers.
Trait-based climate change vulnerability assessments provide another method to evaluate the potential risks to species posed by climate change [21–23]. In general, vulnerability assessments are a formal approach for identifying and prioritizing the vulnerabilities in a system [22, 23]. They often involve expert elicitation to estimate the general sensitivities of species to a stressor. The approach fills the need for broad, transparent, relatively quick evaluation of the vulnerability of multiple species. Vulnerability assessments have been used to evaluate the risk of overfishing for species within given regions [24, 25] and are increasingly being used to evaluate the vulnerabilities of marine species to climate change [26–31].
There are many forms, but in general, trait-based vulnerability assessments identify: i) environmental variables expected to change that could impact species (termed exposure factors) and ii) sensitivity attributes that predict a species intrinsic resilience to change [21, 22, 29, 32]. Some vulnerability assessments separate sensitivity into two components: adaptive capacity and sensitivity [33], but we choose to combine adaptive capacity attributes with sensitivity attributes [34]. Specifically for climate vulnerability, exposure factors include climate variables that have the potential to affect productivity or distribution of a species (or population) in a specific region. For example, temperature is a climate factor that affects species via multiple mechanisms from enzyme reactions to feeding rate to seasonal distribution [35]. Species sensitivity attributes include biological or ecological variables that predict the vulnerability to climate change. For example, a species with an inherently low maximum per capita population growth rate is more sensitive to changes in climate compared to species with an inherently high maximum per capita population growth rate. The exposure factors and sensitivity attributes are scored for each species based on a pre-defined scoring system. These scores are combined across exposure factors and sensitivity attributes to derive a relative species-specific climate vulnerability score. While these methods have limitations, the framework allows a diverse set of species to be assessed in a relatively short period of time, and provides a foundation for further research and management responses [21].
Our objective was to conduct a climate vulnerability assessment for fish and invertebrate species in the Northeast U.S. Continental Shelf Large Marine Ecosystem (hereafter Northeast U.S. Shelf) using the National Marine Fisheries Service (NMFS) Climate Vulnerability Assessment Methodology [34]. We use climate projections between 2005–2055 to evaluate climate change and decadal variability in the 20–40 year time frame. Separating anthropogenic climate change from natural decadal variability is difficult [36] and although we use climate change throughout most of this paper, it is important to recognize that the signals of both change and variability are included in the projections. We define vulnerability as a change in a species’ productivity and or abundance associated with a changing climate, including both climate change and decadal climate variability. We also evaluate the potential for a change in distribution and estimate the directional effect (positive or negative) of a changing climate on species in the Northeast U.S. Shelf. This ecosystem supports valuable commercial ($1.6 billion from landings in 2013 [37]) and recreational ($14.8 billion in total angler expenditures [38]) fisheries. The region is also experiencing relatively rapid climate change [39]. Numerous studies have linked recent climate change to changes in the region’s fish and invertebrate populations, including changes in productivity [4, 8] and distribution [40–43]. Of the numerous fishery species in the region, climate variables have only been directly incorporated into scientific advice and management in a few cases [44, 45]. Although this species-by-species approach is necessary, scientists and managers alike need a broad perspective within which to set research priorities and frame management decisions. Our purpose in conducting this vulnerability assessment is to provide such a system-wide perspective for the Northeast U.S. Shelf.
Materials and Methods
The methods used here, and the development and rationale behind them, are fully described in the National Marine Fisheries Service (NMFS) Climate Vulnerability Assessment Methodology [34] (see Fig 1). The steps are: 1) scoping and planning the assessment including i) identifying the spatial region, ii) the species to include, iii) the climate variables to include as exposure factors, iv) the biological and ecological traits to include as sensitivity attributes, and v) recruiting experts to participate in the assessment; 2) preparation of materials for the assessment including i) consolidating available information on each species, ii) obtaining information on the future state of exposure factors, and iii) providing the spatial overlap between climate exposure and species distributions in the region; 3) expert scoring of the different components of the assessment including i) climate exposure scoring, ii) sensitivity attribute scoring, iii) quantifying expert certainty in scoring, iv) scoring the directional effect of climate change on a species in the region, and v) scoring the quality of data used in the assessment; and 4) analyses of the scores including i) estimating overall climate vulnerability, ii) estimating the potential for a distribution change using a subset of sensitivity attributes, iii) estimating certainty in overall climate vulnerability, potential for a distribution change, and the directional effect of climate change using bootstrapping; iv) identifying the importance of each exposure factor and sensitivity attribute to the overall climate vulnerability using a leave-one out sensitivity analysis, v) evaluating the results on a functional group basis, and vi) developing species specific narratives that summarize the results for each species.
Fig 1. 4 Steps used in the Northeast Fisheries Climate Vulnerability Assessment.
For more details see the NMFS Climate Vulnerability Assessment Methodology [34].
Scoping and Planning
Study Area
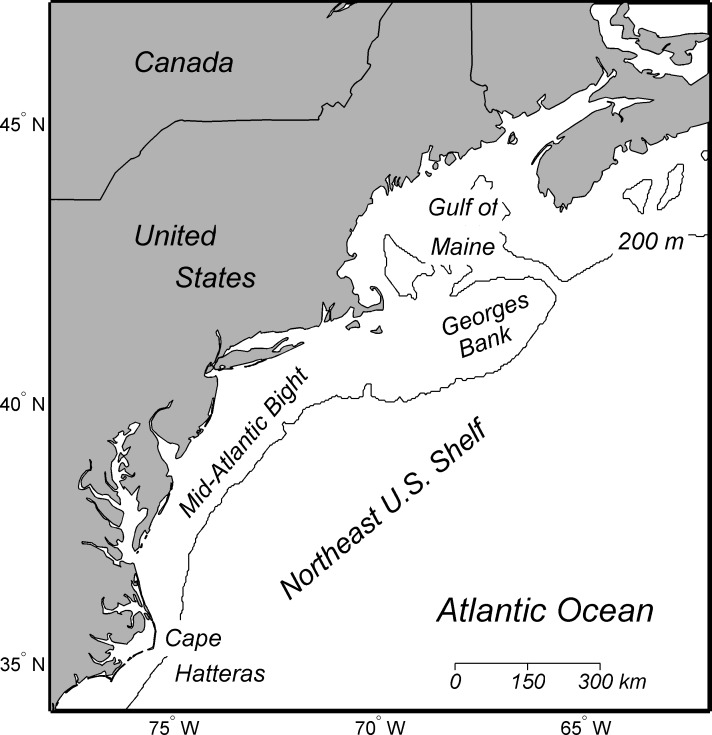
The study area was the Northeast U.S. Shelf, which ranges from Cape Hatteras, North Carolina through the Gulf of Maine (Fig 2). The focus was on species that occur in marine waters of the Northeast U.S. Shelf, but a number of these species use freshwater, estuaries, and offshore areas during some portion of their life history [46]. Thus the study area included freshwater systems, as well as the shelf and oceanic waters.
Fig 2. Map of Northeast U.S. Continental Shelf Large Marine Ecosystem.
Species Included
The focus of the vulnerability assessment was on marine fish and invertebrate species that commonly occur in the Northeast U.S. Shelf, including exploited species (e.g., Atlantic Cod, Atlantic Sea Scallop), protected species (e.g., Atlantic Salmon), and ecologically important species (e.g., Sand Lances). The exploited species included federally and state managed species, as well as species that are not currently managed but are harvested in the region. Federal commercial and recreational landings were evaluated in developing the list of species. Protected fish species were also assessed including fish species listed under the Endangered Species Act [47] and species considered as Species of Concern by NMFS [48]. Several ecologically important forage fish species were included based on species identified as forage by the Mid-Atlantic Fishery Management Council [49]. Highly migratory species were generally excluded because much of their life cycle is completed outside the study area. In total, 82 species were included and divided into 6 functional groups: Coastal Fish (n = 14), Diadromous Fish (n = 10), Elasmobranchs (n = 12), Groundfish (n = 19), Benthic Invertebrates (n = 18), and Pelagic Fish and Cephalopods (n = 9) (Table 1). These functional groups were based in part on phylogeny and in part on habitats occupied.
Table 1. Species included in the Northeast Fisheries Climate Vulnerability Assessment.
Assigned functional group, common name, and scientific name of the 82 fish and invertebrate species included in the Northeast Fisheries Climate Vulnerability Assessment.
| Group | Common Name | Scientific Name |
|---|---|---|
| Coastal Fish | Atlantic Croaker | Micropogonias undulates |
| Coastal Fish | Atlantic Menhaden | Brevoortia tyrannus |
| Coastal Fish | Black Sea Bass | Centropristis striata |
| Coastal Fish | Northern Kingfish | Menticirrhus saxatilis |
| Coastal Fish | Red Drum | Sciaenops ocellatus |
| Coastal Fish | Scup | Stenotomus chrysops |
| Coastal Fish | Spanish Mackerel | Scomberomorus maculatus |
| Coastal Fish | Spot | Leiostomus xanthurus |
| Coastal Fish | Spotted Seatrout | Cynoscion nebulosus |
| Coastal Fish | Striped Bass | Morone saxatilis |
| Coastal Fish | Summer Flounder | Paralichthys dentatus |
| Coastal Fish | Tautog | Tautoga onitis |
| Coastal Fish | Weakfish | Cynoscion regalis |
| Coastal Fish | Winter Flounder | Pseudopleuronectes americanus |
| Diadromous Fish | Alewife | Alosa pseudoharengus |
| Diadromous Fish | Conger Eel | Anguilla oceanica |
| Diadromous Fish | American Eel | Anguilla rostrata |
| Diadromous Fish | American Shad | Alosa sapidissima |
| Diadromous Fish | Atlantic Salmon | Salmo salar |
| Diadromous Fish | Atlantic Sturgeon | Acipenser oxyrhynchus |
| Diadromous Fish | Blueback Herring | Alosa aestivalis |
| Diadromous Fish | Hickory Shad | Alosa mediocris |
| Diadromous Fish | Rainbow Smelt | Osmerus mordax |
| Diadromous Fish | Shortnose Sturgeon | Acipenser brevirostrum |
| Elasmobranchs | Barndoor Skate | Dipturus laevis |
| Elasmobranchs | Clearnose Skate | Raja eglanteria |
| Elasmobranchs | Dusky Shark | Carcharhinus obscurus |
| Elasmobranchs | Little Skate | Leucoraja erinacea |
| Elasmobranchs | Porbeagle | Lamna nasus |
| Elasmobranchs | Rosette Skate | Leucoraja garmani |
| Elasmobranchs | Sand Tiger | Carcharias taurus |
| Elasmobranchs | Smooth Dogfish | Mustelus canis |
| Elasmobranchs | Smooth Skate | Malacoraja senta |
| Elasmobranchs | Spiny Dogfish | Squalus acanthias |
| Elasmobranchs | Thorny Skate | Amblyraja radiata |
| Elasmobranchs | Winter Skate | Leucoraja ocellata |
| Groundfish | Acadian Redfish | Sebastes fasciatus |
| Groundfish | American Plaice | Hippoglossoides platessoides |
| Groundfish | Atlantic Cod | Gadus morhua |
| Groundfish | Atlantic Hagfish | Myxine glutinosa |
| Groundfish | Atlantic Halibut | Hippoglossus hippoglossus |
| Groundfish | Atlantic Wolffish | Anarhichas lupus |
| Groundfish | Cusk | Brosme brosme |
| Groundfish | Haddock | Melanogrammus aeglefinus |
| Groundfish | Monkfish (Goosefish) | Lophius americanus |
| Groundfish | Ocean Pout | Zoarces americanus |
| Groundfish | Offshore Hake | Merluccius albidus |
| Groundfish | Pollock | Pollachius virens |
| Groundfish | Red Hake | Urophycis chuss |
| Groundfish | Silver Hake | Merluccius bilinearis |
| Groundfish | Tilefish | Lopholatilus chamaeleonticeps |
| Groundfish | White Hake | Urophycis tenuis |
| Groundfish | Windowpane | Scophthalmus aquosus |
| Groundfish | Witch Flounder | Glyptocephalus cynoglossus |
| Groundfish | Yellowtail Flounder | Limanda ferruginea |
| Pelagic Fish and Cephalopods | Anchovies | Anchoa hepsetus / Anchoa mitchilli |
| Pelagic Fish and Cephalopods | Atlantic Herring | Clupea harengus |
| Pelagic Fish and Cephalopods | Atlantic Mackerel | Scomber scombrus |
| Pelagic Fish and Cephalopods | Atlantic Saury | Scomberesox saurus |
| Pelagic Fish and Cephalopods | Bluefish | Pomatomus saltatrix |
| Pelagic Fish and Cephalopods | Butterfish | Peprilus triacanthus |
| Pelagic Fish and Cephalopods | Longfin Inshore Squid | Doryteuthis pealeii |
| Pelagic Fish and Cephalopods | Sand Lances | Ammodytes americanus & Ammodytes dubius |
| Pelagic Fish and Cephalopods | Northern Shortfin Squid | Illex illecebrosus |
| Benthic Invertebrates | American Lobster | Homarus americanus |
| Benthic Invertebrates | Atlantic Sea Scallop | Placopecten magellanicus |
| Benthic Invertebrates | Atlantic Surfclam | Spisula solidissima |
| Benthic Invertebrates | Bay Scallop | Argopecten irradians |
| Benthic Invertebrates | Bloodworm | Glycera dibranchiata |
| Benthic Invertebrates | Blue Crab | Callinectes sapidus |
| Benthic Invertebrates | Blue Mussel | Mytilus edulis |
| Benthic Invertebrates | Cancer Crabs | Cancer borealis / Cancer irroratus |
| Benthic Invertebrates | Channeled Whelk | Busycotypus canaliculatus |
| Benthic Invertebrates | Deep-sea Red Crab | Chaceon quinquedens |
| Benthic Invertebrates | Eastern Oyster | Crassostrea virginica |
| Benthic Invertebrates | Green Sea Urchin | Strongylocentrotus droebachiensis |
| Benthic Invertebrates | Horseshoe Crab | Limulus polyphemus |
| Benthic Invertebrates | Knobbed Whelk | Busycon carica |
| Benthic Invertebrates | Northern Shrimp | Pandalus borealis |
| Benthic Invertebrates | Ocean Quahog | Arctica islandica |
| Benthic Invertebrates | Northern Quahog | Mercenaria mercenaria |
| Benthic Invertebrates | Softshell Clam | Mya arenaria |
Climate Exposure Factors
Exposure is a measure of the projected magnitude of change in the physical environment due to climate [23, 29]. Exposure factors are those climate variables included in the assessment that could impact a species (e.g., temperature, salinity). The exposure score includes information about the magnitude of the expected climate change, but not in relation to each species’ tolerances, which are often unknown. Exposure factors were chosen based on two criteria. First, factors were chosen on the basis that studies have found an effect on fish and invertebrate species in the Northeast U.S. Shelf. Second, factors were chosen that are likely to be well represented in the current class of global climate models (models are described below) [17]. Seven factors were selected: ocean surface temperature (upper 10 m), ocean surface salinity (upper 10 m), surface air temperature, precipitation, surface pH (upper 10 m), currents, and sea-level rise. Bottom estimates were not used owing to the low spatial resolution of current climate models and the fact that most of the models used do not resolve the bathymetry of the Northeast U.S. Shelf. Similarly, primary productivity was not used because of the importance of regional-scale oceanography and estuaries, neither of which are resolved in the current ensemble of current global climate models. All factors were equally weighted owing to the limited knowledge regarding the magnitude of effects and species responses to climate. Changes in the mean and variance of ocean temperature, ocean salinity, air temperature, precipitation, and pH were included, whereas only changes in mean sea level and ocean currents were considered resulting in a total of 12 climate exposure factors (Table 2). Ocean temperature is an important climate factor, which numerous studies have linked to changes in distribution and productivity [4, 41, 42, 50]. Fewer studies have linked changes in ocean salinity to biological responses, but changes in salinity may increase metabolic costs [51], reducing resilience to other changes. Salinity also affects stratification, mixing, and thus the timing and magnitude of primary production [52]. Most climate models are at a scale where water temperatures in estuaries and freshwater areas are not resolved, so air temperature is used as a proxy [14, 53], as it is directly linked to the temperature in shallow water owing to air-water heat exchange [4]. Air temperature and surface ocean temperature are likely correlated because of the large-scale of climate induced warming, but the two factors are important and distinct in terms of their impact on the biology of some species (e.g., Atlantic Salmon are exposed to climate impacts both in freshwater and marine habitats). Streamflow is linked to productivity of a number of diadromous species [54, 55]. Most climate models do not simulate streamflow, rather they have river routing systems that move water among model grid cells. Precipitation is therefore used here as a proxy of the amount of water in streams and rivers. Numerous studies have found an effect of increased dissolved CO2 (ocean acidification) on marine organisms from larval survival in molluscs to olfaction in fish [56, 57]. Estimates of pH were derived from Earth Systems Models, which simulate the carbon system to varying degrees of complexity. Ocean currents were also used as a factor since most marine organisms have planktonic early life stages that rely on advection for transport to habitats necessary for the continuation of the life cycle [58]. Small-scale changes in currents cannot be assessed from the current global climate models, but changes in large scale changes can be considered. Sea-level rise threatens a variety of coastal habitats including marshes, seagrass beds, and beaches [59, 60]. This threat is exacerbated by the large degree of coastal development in the Northeast U.S. Shelf [61] and the large reliance of fish and invertebrate species on coastal habitats during portions of their life history [46].
Table 2. Climate Exposure Factors and Sensitivity Attributes.
List of climate exposure factors and sensitivity attributes used in the climate vulnerability assessment. See NMFS Climate Vulnerability Assessment Methodology for more details [34].
| Climate Factor or Biological Attribute | Goal | Low Score | High Score | |
|---|---|---|---|---|
| Climate Factors | ||||
| Mean Ocean Surface Temperature | To determine if there are changes in mean ocean surface temperature comparing the 1956–2005 to 2006–2055 periods | Low magnitude of change | High magnitude of change | |
| Mean Ocean Surface Salinity | To determine if there are changes in mean ocean surface salinity comparing the 1956–2005 to 2006–2055 periods | Low magnitude of change | High magnitude of change | |
| Mean Air Temperature | To determine if there are changes in mean air temperature comparing the 1956–2005 to 2006–2055 periods. Air temperature is a proxy for water temperatures in lakes, streams, river, estuaries, and nearshore areas | Low magnitude of change | High magnitude of change | |
| Mean Precipitation | To determine if there are changes in mean precipitation comparing the 1956–2005 to 2006–2055 periods. Precipitation is a proxy for streamflow. | Low magnitude of change | High magnitude of change | |
| Mean Ocean pH | To determine if there are changes in mean ocean pH comparing the 1956–2005 to 2006–2055 periods. pH represents ocean acidification. | Low magnitude of change | High magnitude of change | |
| Variability in Ocean Surface Temperature | To determine if there are changes in variability of ocean surface temperature comparing the 1956–2005 to 2006–2055 periods | Low magnitude of change | High magnitude of change | |
| Variability in Ocean Surface Salinity | To determine if there are changes in variability of ocean surface salinity comparing the 1956–2005 to 2006–2055 periods | Low magnitude of change | High magnitude of change | |
| Variability in Air Temperature | To determine if there are changes in variability of air temperature comparing the 1956–2005 to 2006–2055 periods. Air temperature is a proxy for water temperatures in lakes, streams, river, estuaries, and nearshore areas | Low magnitude of change | High magnitude of change | |
| Variability in Precipitation | To determine if there are changes in variability of precipitation comparing the 1956–2005 to 2006–2055 periods. Precipitation is a proxy for streamflow. | Low magnitude of change | High magnitude of change | |
| Variability in pH | To determine if there are changes in variability of ocean pH comparing the 1956–2005 to 2006–2055 periods. pH represents ocean acidification. | Low magnitude of change | High magnitude of change | |
| Sea Level Rise | To evaluate the magnitude of sea level rise relative to the ability of nearshore habitats to change | Low magnitude of change | High magnitude of change | |
| Ocean Currents | To evaluate changes in large-scale circulation. | Low magnitude of change | High magnitude of change | |
| Biological Attributes | ||||
| Prey Specificity | To determine, on a relative scale, if the stock is a prey generalist or a prey specialist. | Prey generalist | Prey specialist | |
| Habitat Specificity | To determine, on a relative scale, if the stock is a habitat generalist or a habitat specialist while incorporating information on the type and abundance of key habitats. | Habitat generalist | Habitat specialist | |
| Sensitivity to Ocean Acidification | To estimate a stock’s sensitivity to ocean acidification based on its relationship with “shelled species.” (followed Kroeker et al. 2012) | Sensitive taxa | Insensitive taxa | |
| Complexity in Reproductive Strategy | To determine how complex the stock’s reproductive strategy is and how dependent reproductive success is on specific environmental conditions. | Low compleixty, broadcast spawning | High complexity; aggregate spawning | |
| Sensitivity to Temperature | To use the distribution of the species as a proxy for its sensitivity to temperature. Note: that this attribute uses species (vs. stock) distributions as they better predict thermal requirements. | Broad thermal limits | Narrow thermal limits | |
| Early Life History Survival and Settlement Requirements | To determine the relative importance of early life history requirements for a stock. | Generalist with few requirements | Specialists with specific requirements | |
| Stock Size/Status | To estimate stock status to clarify how much stress from fishing the stock is experiencing and to determine if the stock’s resilience or adaptive capacity are compromised due to low abundance. | High abundance | Low abundance | |
| Other Stressors | To account for conditions that could increase the stress on a stock and thus decrease its ability to respond to changes. | Low level of other stressors | High level of other stressors | |
| Population Growth Rate | To estimate the relative productivity of the stock. | High population growth | Low population growth | |
| Dispersal of Early Life Stages | To estimate the ability of the stock to colonize new habitats when/if their current habitat becomes less suitable. | High dispersal | Low dispersal | |
| Adult Mobility | To estimate the ability of the stock to move to a new location if their current location changes and is no longer favorable for growth and/or survival. | High mobility | Low mobility | |
| Spawning Cycle | To determine if the duration of the spawning cycle for the stock could limit the ability of the stock to successfully reproduce if necessary conditions are disrupted by climate change. | Year-round spawning | One event per year | |
Sensitivity Attributes
Sensitivity attributes represent biological traits that are indicative of an ability or inability of a species to respond to environmental change. All 12 attributes defined in the NMFS Climate Vulnerability Assessment Methodology [34] were used here (Table 2). As an example, the Adult Mobility of a clam is low and as a result, adults would be unable to move as climate changes making them more vulnerable to climate change. The definition for a few sensitivity attributes varies slightly from those presented in the NMFS Methodology [34] (see S1 Supporting Information) due to minor changes in the definitions in the methodology, which occurred after the implementation described here.
Participants
The expert group consisted of the core development team for the methodology [34], as well as regional experts from NOAA NMFS including stock assessment scientists, fisheries scientists, ecologists, and oceanographers. Fourteen experts participated: two experts per functional group (Coastal Fish, Benthic Invertebrates, Pelagic Fish and Cephalopods, Elasmobranchs, and Diadromous Fish), with the exception of 4 experts for Groundfish. Most experts have experience with species in several functional groups. Two climate experts also participated, providing access and advice regarding the climate exposure factors. Representatives of the New England Fisheries Management Council, Mid-Atlantic Fisheries Management Council and Greater Atlantic Regional Fisheries Office provided input on species at a workshop where the experts met to discuss exposure and sensitivity scoring (see expert certainty below).
Assessment Preparation
Species Profiles
Species profiles were prepared to summarize the biological and ecological information needed for experts to score the sensitivity attributes. The consolidation of the information was an important step to ensure that all experts were provided with the same baseline information. In general, the Northeast U.S. Shelf is data rich with regards to fish and invertebrate biology and ecology. However, there are species included in this assessment that are considered data-poor and a Data Quality attribute was included to help identify information gaps. Numerous summary documents were available to complete species profiles including stock assessments [62, 63], Essential Fish Habitat source documents [64], and monographs that describe the biology and ecology of fish and invertebrate species in the region [46, 65, 66]. Peer-reviewed literature was also used to complete the profiles for some species when information was not summarized in the documents described above. General guidelines were developed for completing species profiles and approximately 3–6 hours were spent on each species profile. One scientist then reviewed all the profiles to ensure consistency in content.
Climate Projections
For most climate factors included in the assessment, exposure was estimated from an ensemble of global climate models used in the Intergovernmental Panel on Climate Change Assessment Report 5 (IPCC AR5). As described above, the choice of climate factors to use in this assessment was impacted by the resolution of the models used for projections. The ocean resolution of these models is too course (0.5–1.0° latitude, 1.0–1.5° longitude) to allow mesoscale eddies, sub-mesoscale eddies, fronts, and regional scale bathymetry and circulation [67]. The Representative Concentration Pathway 8.5 (RCP 8.5) was used, which represents a “business-as-usual” scenario assuming little to no stabilization of greenhouse gas emissions by 2100 [68, 69]. The 2006–2055 period was chosen for projections because this represents the coming decades, which are of more relevance to living marine resource management than the end of the century. The 50 year average focuses the projections on the forced climate change signal, but also includes the multi-decadal variability signal [17, 36]. The exposures to ocean surface temperature (upper 10 m), surface air temperature, ocean surface salinity (upper 10 m), and precipitation were estimated from an ensemble of 25–35 global climate models. Exposure to ocean acidification was estimated from an ensemble of 11 earth system models. These ensembles were defined as those models available on the Earth System Grid Federation Portal (http://pcmdi9.llnl.gov/esgf-web-fe/) in autumn 2013 that simulated a given parameter over the time period of interest.
Using these model outputs, the change in climate conditions in the future relative to the past were expressed in a standard deviate framework (for the mean factors) and an F-test framework (for the variance factors). Maps of these standard deviates and variance ratios were obtained from NOAA’s Ocean Climate Change Web Portal [70]. Maps of inter-model variability were also obtained to assess the among-model uncertainty. The approach scales projected future change to the past mean state and variability in that state (i.e., the standard deviate of change is used, not the mean change). For example, the exposure resulting from 1°C increase is greater in an ecosystem with a 0.2°C standard deviation in past temperatures compared to a system with a 2°C standard deviation in past temperatures. This construct is based on macroecological principals that indicate niches are broader in areas of more variable environment [71, 72].
Exposure to change in currents and sea-level rise was evaluated by a review of the literature. A summary of likely changes in currents was prepared in consultation with oceanographers and climate scientists (S2 Supporting Information). Exposure to a change in currents was scored based on a species use or dependence of large-scale currents in the region. For example American Eel is dependent on the Gulf Stream to transport larvae from spawning locations in the Sargasso Sea to the oceanic waters off the Northeast U.S. Shelf. Thus American Eel is exposed to changes in the Gulf Stream. Similarly for Sea-Level Rise, a summary was prepared of the expected rate of change of regional sea level compared to rates of sea level rise that coastal habitats can accommodate (S3 Supporting Information). Exposure to sea-level rise was scored based on a species’ reliance on wetland, seagrass, and estuarine habitats.
Species distributions
Species distribution information was obtained primarily through the Ocean Biogeographic Information System (OBIS) [73]. OBIS data is point occurrence data only, however, the Northeast U.S. Shelf is well represented in OBIS through the inclusion of data from several ecosystem-wide surveys. For some species, information obtained through OBIS was supplemented with information from other sources including stock assessments [62, 63], Essential Fish Habitat source documents [64], and monographs [46, 65, 66]. Owing to the multiple sources of information used for species distribution, a quantitative match between exposure and distribution was not calculated and experts were allowed to judge spatial overlap individually using the information provided. The uncertainty introduced by expert elicitation is small since the Northeast U.S. Shelf is well sampled and species distributions are relatively well known [41, 42].
Scoring
Climate Exposure
Four scientists scored climate exposure in consultation with a broader group of climate scientists. Prior to scoring, the experts received an overview of the output of the climate model ensembles [70]. Experts then used information on species distribution and the exposure maps of ocean surface temperature, ocean surface salinity, air temperature, and pH to estimate exposure in the Northeast U.S. Shelf ecosystem. The comparisons between species distribution and exposure maps were made visually by each expert independently. This species specific exposure was then converted to a score using pre-defined criteria (S4 Supporting Information). Scores for exposure were: low, moderate, high, and very high. If a species was not exposed to a given factor, it was scored as low exposure (e.g., Acadian Redfish is not exposed to changes in precipitation because they live in deep, offshore waters). For sea-level rise and ocean currents, which were not included in the climate model output, the experts reviewed the exposure summaries (S2 Supporting Information, S3 Supporting Information) and then discussed these documents as a group. Species with no exposure to change in currents were scored as low and species with exposure to currents were scored on the basis of the magnitude of change estimated by expert opinion (clarified in S2 Supporting Information). With regard to Sea-Level Rise, species with no reliance on nearshore habitats were scored low and species that use these habitats were scored based on the magnitude of the impacts of sea-level rise estimated by expert opinion (clarified in S3 Supporting Information).
Sensitivity Attributes
Fourteen experts scored sensitivity attributes. Benthic Invertebrate, Coastal Fish, Pelagic Fish and Cephalopods, Elasmobranchs, and Diadromous Fish group experts scored all the species in their assigned group and a random selection of other species. Owing to the number of Groundfish species, assigned experts scored half the Groundfish species and a random selection of other species. Experts used the species profiles and attribute descriptions (S1 Supporting Information) as a common baseline, but were encouraged to bring their expertise and new information to the scoring process.
Expert Certainty
Scoring of both climate exposure and biological sensitivity was completed individually using a 5 tally scoring system. Each expert had 5 tallies to score each exposure factor or sensitivity attribute; they could place all five tallies in the same bin (e.g., low) for attributes or factors with high certainty, or they could spread their tallies across all bins for attributes or factors with less certainty (e.g., low, moderate, high, very high). Once the individual scores were recorded, experts met in person and discussed scores as a group. Experts were given the opportunity to independently change their final scores based on the discussion, however, there was no requirement nor expectation that experts reach consensus. The full results of the expert scoring- number of tallies per scoring bin (low, moderate, high, very high) by species and attribute are provided in S5 Supporting Information, S1 Dataset, and S2 Dataset.
Directional Effect
Experts were asked to score the directional effect of climate change for each species, giving an overall indication whether impacts are anticipated to be negative, neutral, or positive on the species in the region. For each species, 3 experts scored directional effect. Experts included the functional group experts for each species (n = 2) and the lead author. Each expert was given 4 tallies to score in the 3 bins. The scores were converted to numbers (negative = -1, neutral = 0, positive = 1) and a weighted average was calculated based on the total of 12 tallies. Weighted averages below -0.333 were classified as an overall negative effect, weighted averages between -0.333 and 0.333 were classified as an overall neutral effect, and weighted averages above 0.333 were classified as an overall positive effect. The full results of the expert scoring, number of tallies per scoring bin (Negative, Neutral, Positive) by species are provided in S5 Supporting Information (and S1 Dataset and S2 Dataset).
Data Quality
Experts also assessed the quality of information available for scoring (i.e., data quality, S6 Supporting Information). Each expert noted their opinion of data quality for each sensitivity attribute or exposure factor for each species. These data quality scores were then averaged across experts and the proportion of data quality scores <2 for each species was calculated; a score of 2 represents limited data available.
Analyses
Five analyses were conducted on the expert scores of climate exposure, sensitivity attributes, and directional effect. 1) Overall climate vulnerability was calculated from a combination of climate exposure factors and biological sensitivity attributes. 2) Potential for a change in species distribution was calculated using a subset of sensitivity attributes. 3) Bootstrap analyses were used to evaluate the certainty in the overall vulnerability, potential for a change in distribution, and directional effect of climate change. 4) Leave-one-out sensitivity analyses were used to evaluate the effects exposure factors and sensitivity attributes in determining overall vulnerability. 5) Overall climate vulnerability, potential for distribution change, and directional effect of climate change were analyzed by functional groups to identify larger patterns in climate effects.
Estimate of Overall Vulnerability
Overall climate vulnerability was estimated using a four step process. First, the component scores (low, moderate, high and very high) were assigned a numerical value (1, 2, 3, and 4). Second, an average score for each climate exposure factor and sensitivity attribute was calculated as the weighted-mean of the experts’ tallies. Third, an overall exposure and sensitivity score was calculated from the weighted means using a logic rule (Table 3). Fourth, an overall climate vulnerability score was calculated by multiplying the overall exposure and sensitivity scores. The product of the two component numeric scores results in a value between 1 and 16. The overall climate vulnerability rank is then classified as follows: 1–3 low, 4–6 moderate, 8–9 high, and 12–16 very high.
Table 3. Logic rule for calculating overall species’ climate exposure and biological sensitivity.
The scoring rubric is based on a logic model where a certain number of individual scores above a certain threshold are used to determine the overall climate exposure and overall biological sensitivity.
| Overall Sensitivity or Exposure Score | Numeric Score | Logic Rule |
|---|---|---|
| Very High | 4 | 3 of more attributes or factors mean ≥ 3.5 |
| High | 3 | 2 of more attributes or factors mean ≥ 3.0 |
| Moderate | 2 | 2 of more attributes or factors mean ≥ 2.5 |
| Low | 1 | All other scores |
Potential for Distribution Change
High potential for change in species distribution was defined as highly mobile adults, broadly dispersing early life stages, low habitat specificity, and high temperature sensitivity [34]. These attributes have some skill in predicting species that can change distribution in response to climate change; increased dispersal capacity and ecological generalism promote changes in distribution [74]. The potential for a change in species distribution was estimated using these attributes, reversing the scores for Adult Mobility, Early Life Stage Dispersal, and Habitat Specificity, and applying the same logic rule as in the general vulnerability calculation.
Certainty in Vulnerability Scores
Bootstrap analysis was used to calculate the certainty of the climate vulnerability scores, potential for change in distribution scores and directional effect scores. Using the climate vulnerability scores as the examples, the scores across all experts for a given exposure factor (n = 20; 4 experts and 5 tallies) or sensitivity attribute (n = 25; 5 experts and 5 tallies) were drawn randomly with replacement. This was repeated 10,000 times for each of the 12 sensitivity attributes and the 12 exposure factors, and the overall vulnerability score was calculated for each iteration. The outcomes of each iteration was recorded and the proportion of these 10,000 repetitions that scored in each overall vulnerability bin was enumerated. A similar bootstrapping analysis was conducted on the potential for a distribution change (n = 25; 5 experts and 5 tallies) and the directional effect scoring (n = 12; 3 experts and 4 tallies).
Importance of Climate Exposure Factors and Sensitivity Attributes
For the sensitivity analysis, the overall vulnerability score for each species was calculated leaving out the scores for each sensitivity attribute or exposure factor. These analyses were then evaluated across species to determine influential factors and attributes in the overall vulnerability rank.
Functional Group Evaluation
To evaluate the similarity of vulnerability across functional groups, overall climate vulnerability, potential for distribution change, and directional effect of climate change were pooled by functional group. In addition, sensitivity attribute scores within and among functional groups was evaluated using non-metric multidimensional scaling.
Species Narratives
In addition to the composite results that show the relative vulnerabilities across species, species specific vulnerability narratives were prepared (S7 Supporting Information). These narratives provide the distribution of tallies and the data quality score for each exposure factor and sensitivity attribute, the overall climate vulnerability, potential for distribution change, directional effect scores, and the certainty in these scores. Additionally, a summary of identified climate effects on the species and a synopsis of the life history is given.
Ethics Statement
This study was not based on Human Subject Research. The study was not based in Animal Research. No field permits were involved. This was an expert opinion vulnerability assessment and all experts involved are co-authors on the paper.
Results
Overall Climate Vulnerability
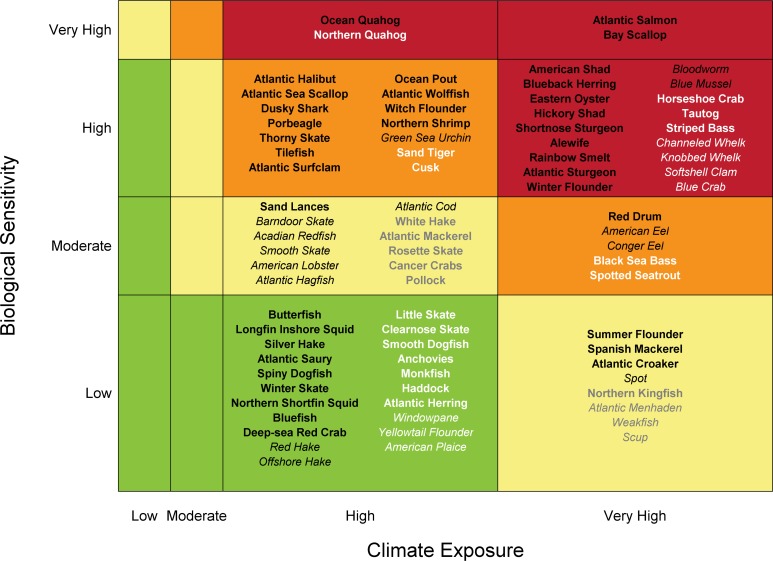
The 82 species were nearly equally split among the different climate vulnerability ranks: very high (~27%), high (~23%), moderate (~24%) and low (~26%) (Fig 3). Climate exposure scores for all 82 species were high or very high indicating the magnitude of climate change relative to the variability of past conditions is high. Biological sensitivity ranged from low to very high. The certainty in the score of the majority of species exceeded 90% based on the bootstrap analysis (Fig 3). Approximately 27% of species had certainty scores between 66–90%. Approximately 12% of species had certainty scores <66%. For certainty scores less than 50%, a majority of bootstrapped climate vulnerability scores were different than the actual score. Species narratives provide species-specific summaries of the results (S7 Supporting Information).
Fig 3. Overall climate vulnerability score.
For species names and functional groups see Table 1. Overall climate vulnerability is denoted by color: low (green), moderate (yellow), high (orange), and very high (red). Certainty in score is denoted by text font and text color: very high certainty (>95%, black, bold font), high certainty (90–95%, black, italic font), moderate certainty (66–90%, white or gray, bold font), low certainty (<66%, white or gray, italic font).
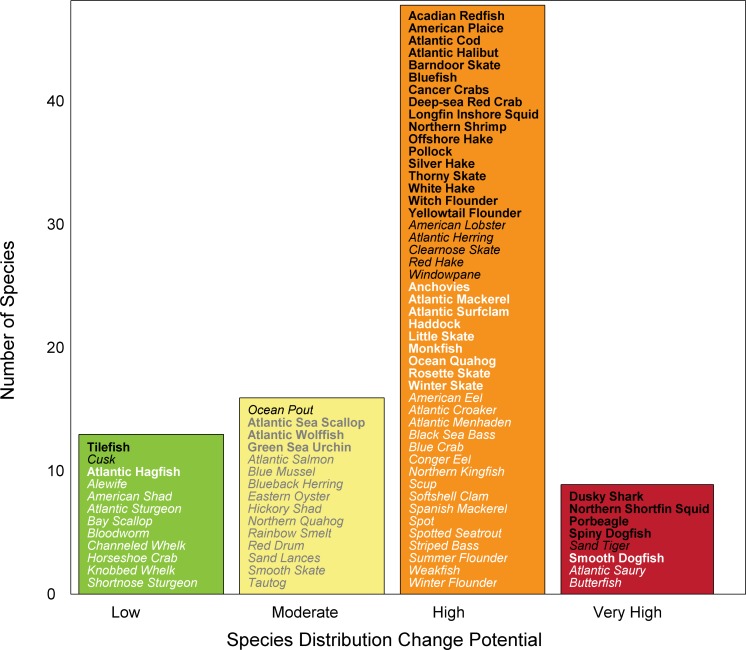
Potential for Distribution Change
Many species in the Northeast U.S. Shelf have life history attributes that suggest distribution may change in response to climate change (Fig 4). More than 50% of the species exhibit very high or high potential for a change in species distribution. In general, overall climate vulnerability (changes in population productivity) varies inversely to the potential for a change in species distribution: species highly vulnerable to a change in productivity have a lower potential to change distribution and vice versa (S8 Supporting Information). However, the certainty potential for distribution change score of almost half the species was <66%; this lower value of certainty compared to overall climate vulnerability results from the lower number of attributes included in the potential for distribution change calculation.
Fig 4. Potential for a change in species distribution.
Potential was calculated using a subset of sensitivity attributes. Colors represent low (green), moderate (yellow), high (orange) and very high (red) potential for a change in distribution. Certainty in score is denoted by text font and text color: very high certainty (>95%, black, bold font), high certainty (90–95%, black, italic font), moderate certainty (66–90%, white or gray, bold font), low certainty (<66%, white or gray, italic font).
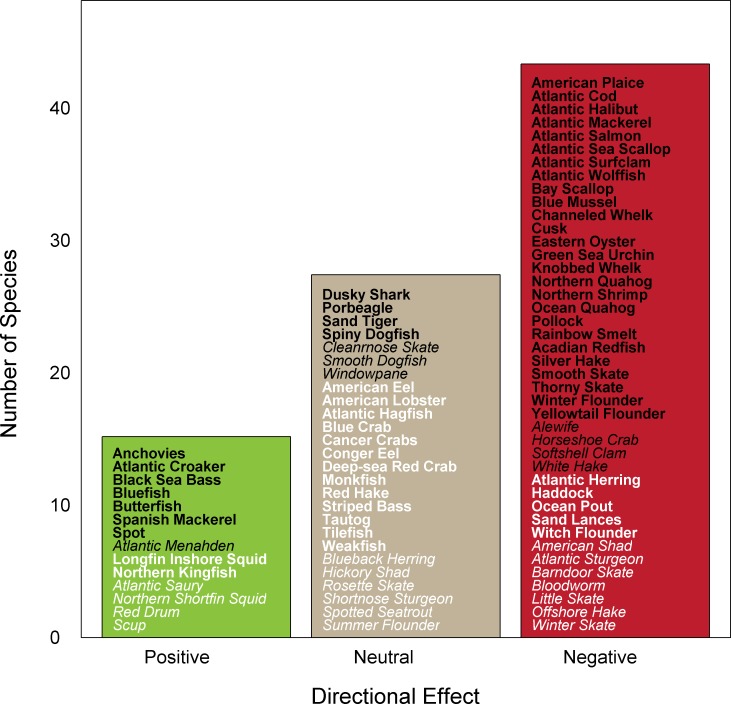
Directional Effect of Climate Change
Based on expert opinion of the directional effect of climate change, approximately half of the species were assessed to be negatively affected by climate change in the Northeast U.S. Shelf (Fig 5). Negative impacts are estimated for many of the iconic species in the ecosystem including Atlantic Sea Scallop, Atlantic Cod, and Atlantic Mackerel. In general, negative effects are anticipated for a number of Benthic Invertebrate and Groundfish species. However, positive effects are anticipated for 17% of species including Inshore Longfin Squid, Butterfish, and Atlantic Croaker. The certainty in directional effect score varied, but was >95% for almost half of the species assessed. However, there were species with low certainty (<66%) in all three directional effect categories. By comparing across the climate vulnerability, potential for distribution change, and directional effect scores, species can be identified that are likely to increase in productivity (e.g., Black Sea Bass) or shift into the region (e.g., Atlantic Croaker) or that are likely to decrease in productivity (e.g., Winter Flounder) or shift out of the region (e.g., Atlantic Mackerel).
Fig 5. Directional effect of climate change.
Colors represent expected negative (red), neutral (tan), and positive (green) effects. Certainty in score is denoted by text font and text color: very high certainty (>95%, black, bold font), high certainty (90–95%, black, italic font), moderate certainty (66–90%, white or gray, bold font), low certainty (<66%, white or gray, italic font).
Evaluation of Exposure Factors and Sensitivity Attributes
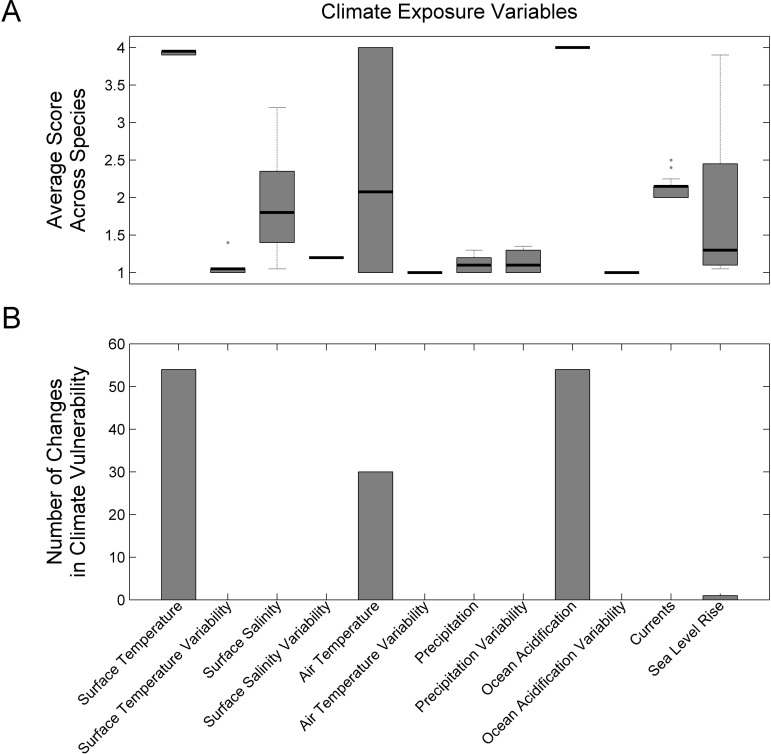
Mean ocean surface temperature change (upper 10 m) and mean surface pH change were determined to be important factors in the climate vulnerability scores (Fig 6). These factors were scored as very high exposure for all species owing to the magnitude of change projected by 2055 (S9 Supporting Information). Mean surface air temperature change (as a proxy for shallow water temperatures) and to a lesser extent sea-level rise were also important for species that were exposed to these factors (Coastal Fish and Diadromous Fish species). Exposure factors that were not as important in determining vulnerability scores exhibited a lower magnitude of change, particularly the variance of the exposure factors and mean changes in precipitation and ocean surface salinity.
Fig 6. Climate exposure factors.
Average climate exposure scores across all species (A) and results of sensitivity analysis for the effect of individual exposure factors on overall climate vulnerability (B).
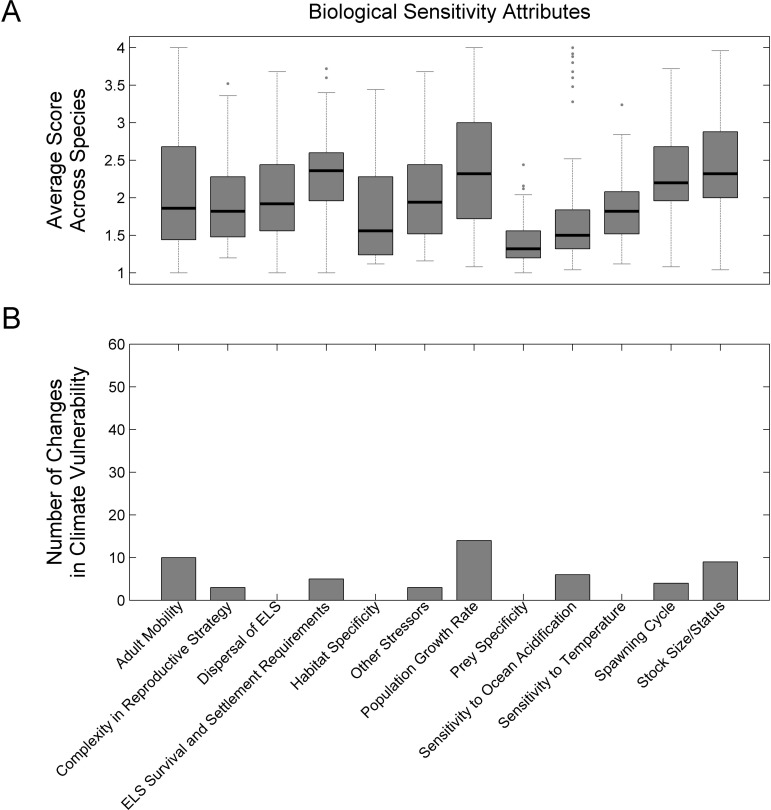
The importance of sensitivity attributes varied across species and there was no subset of dominant attributes (Fig 7). All attributes were scored as very high or high sensitivity for at least one species. There were three attributes that had the strongest influence on climate vulnerability: Population Growth Rate, Adult Mobility, and Stock Status. Removal of the attributes in the sensitivity analysis changed the scores of 14, 10, and 9 species, respectively.
Fig 7. Biological sensitivity attributes.
Average sensitivity attribute scores across all species (A) and results of sensitivity analysis for the effect of individual sensitivity attributes on overall climate vulnerability scores (B).
Functional Group Results
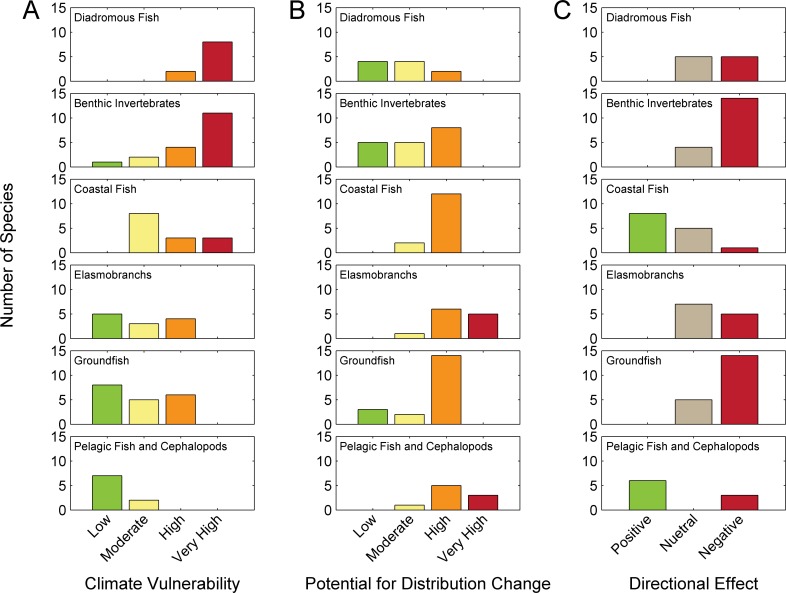
The effects of climate change exhibited some consistency across functional groups. In terms of overall climate vulnerability, Diadromous Fish and Benthic Invertebrate species had the highest vulnerabilities (Fig 8). Some Coastal Fish species also scored very highly vulnerable to climate change. Elasmobranchs and Groundfish groups had no species that scored very highly vulnerable, and Pelagic Fish and Cephalopod species had no species that scored very highly or highly vulnerable. Potential for distribution change also varied by functional groups (Fig 8). Only Pelagic Fish and Cephalopods and Elasmobranchs had very high potential for a distribution change. Diadromous Fish, Benthic Invertebrates, and Groundfish had species with a low potential for distribution change. Similarly, the directional effect of climate change varied across functional groups. All groups had species where negative effects of climate change are estimated and Benthic Invertebrates and Groundfish had greatest proportion of species with estimated negative effects. Only Coastal Fish and Pelagic Fish and Cephalopods had species where positive effects of climate change are estimated within the Northeast U.S. Shelf ecosystem. MDS ordination of sensitivity attributes exhibited some consistency within functional groups but also demonstrated that certain species have attribute that are more similar to species in other functional groups (S10 Supporting Information).
Fig 8. Functional groups and climate vulnerability.
Number of species from each functional group by overall climate vulnerability (A), potential for distribution change (B), and directional effect of climate change (C).
Discussion
The results of this assessment indicate that a number of fish and invertebrate species in the Northeast U.S. Shelf are highly or very highly vulnerable to climate change and decadal scale variability (Fig 3). Climate vulnerability here is defined as the extent to which abundance or productivity of a species could be impacted by climate change. This vulnerability results from the relatively large magnitude of climate change projected for the region over the next 35 years (S9 Supporting Information), as well as attributes of individual species. Changes in population productivity have already been observed in the system [4, 8, 44] and the results presented here suggest that these changes are likely to continue in the future, possibly becoming widespread among species in the ecosystem. The results of this assessment also suggest a large number of species in the ecosystem have a high potential for a change in distribution (Fig 4). Distribution changes have already been observed in the system [41, 42, 75] and have been linked to climate variables and fishing [76]. Finally, this assessment suggests that approximately half of the species included will be affected negatively by climate impacts in the region (e.g., decreased productivity, distribution shifts out of the system). However, positive effects of climate change are expected for some fish and invertebrate species in the ecosystem.
Ocean temperatures, shallow-water temperatures, and ocean acidification were the climate exposure factors with the largest magnitude of change expected by 2055, thereby contributing most to the high and very high climate exposure. There is more known about the impacts of temperature than the impacts of ocean acidification on the species in the NE LME and continued research on the effects of these factors should be a priority. The effects of temperature on species biology and ecology are well documented and there are numerous examples of incorporating temperature into models of population abundance and distribution [11, 14, 77]. These climate induced changes can result in changing reference points for management [11, 14, 78] and changes in stock distribution, which can also influence management [79]. Given the temperature changes experienced in recent decades and projected for the future in the region [39, 80, 81], temperature should be included in regional scientific advice and management. Further, research must continue to understand the mechanisms by which temperature affects species and to parameterize these affects for inclusion in population and ecosystem models [82–84].
The effects of ocean acidification on species biology and ecology are not as well understood [85]. In recent years, a number of studies have started to fill this gap for species in the Northeast U.S. Shelf [57, 86]. However, it is often difficult to place the impacts identified in experimental studies into the context of the magnitude of projected change. In the Northeast U.S. Shelf, pH is projected to decrease by 0.08 to 0.12 units by 2055 (S9 Supporting Information). Many of the studies however use treatments equivalent to ocean acidification conditions expected by 2100 or 2200. In addition, many studies use constant ocean acidification levels, but there is a large magnitude of daily, seasonal, interannual, and regional variability [87]. Research is needed that tests the impacts of ocean acidification at the levels expected over the next 20–40 years to support shorter term projections that can be used in management. One example is a recent study with Atlantic Sea Scallop where fishery yields are projected to decrease in the coming decades owing to the effects of ocean acidification [16]. Because a meta-analysis shows consistent negative impacts of ocean acidification on mollusc larvae [85] this methodology scored molluscs as very sensitive to ocean acidification (S1 Supporting Information). As these species also exhibit low adult mobility, molluscs were ranked with a high or very high vulnerability to climate change. This should be interpreted cautiously, and highlights the need for more research on species specific impacts of ocean acidification. It is imperative to experimentally examine the effect of ocean acidification on exploited species, incorporate this information into future climate vulnerability assessments, and include these effects in assessment models that evaluate the impact of acidification in the coming decades.
In contrast to the high influence of a limited number of exposure factors, the importance of biological sensitivity attributes was more variable; indicating the diversities in life history strategies among fish and invertebrates species in the Northeast U.S. Shelf. In general, Stock Status, Population Growth Rate, and Adult Mobility were the most influential attributes. Improved understanding of these attributes is identified as priority research areas. There are a number of other species specific needs that are identified in the species narratives (S7 Supporting Information). There is also a need to understand the relative role of climate change in concert with multiple other stressors in affecting population abundance. Here we focused on changing climate but included fishery status and other stressors as sensitivity attributes; stress from climate change will act in concert with other stressors. Including multiple stressors as exposure factors is possible in the vulnerability assessment framework [21, 23]; for management to result in long-term sustainability, the important stressors need to be identified and included in management considerations [88]. Fishing is still a dominant factor, but as fishing mortality decreases, the relative importance of other stressors (e.g., climate change, habitat alterations) will increase [8, 76]. New contaminant and disease concerns are being identified and require more study to understand their effect on fish and shellfish populations [89, 90]. In addition, although barriers to diadromous fish passage are decreasing, a number of barriers remain [91].
For this study, adaptive capacity was incorporated into the sensitivity attributes. Adaptive capacity includes a species ability to move when conditions change (adult dispersal), ability to acclimate to changes (plasticity in response, generalist versus specialist), and the ability to evolve as a population or species [33, 92]. This methodology incorporated aspects of the first two, but not the third; the genetic ability to adapt to climate change remains under-known [33, 92]. Understanding genotypic and phenotypic adaptive capacity in marine fish and invertebrates should be a priority for future research.
The results from this assessment can be compared with detailed studies examining past changes in fish and invertebrate species and projecting future changes. In some cases, these more detailed studies seemingly contradict the results presented here. Population productivity of both the Southern New England Yellowtail Flounder and Winter Flounder stocks has decreased and these decreases have been attributed to changes in the environment [8, 44]. In this climate vulnerability assessment, Yellowtail Flounder was ranked as having a low vulnerability to change in population productivity and Winter Flounder was ranked as having a very high vulnerability to changes in productivity. Similarly, Alewife and American Shad have exhibited some of the greatest shifts in distribution in the ecosystem [41], but the potential for a change in species distribution was low. The results of an expert-based assessment are never going to completely agree with the results of more detailed, empirical and process-oriented studies or assessments. However, expert opinion summarizes current knowledge in a defined framework and can guide future monitoring, research, and modeling studies. Further, the studies described above are not directly comparable with the results of this assessment. The changes in productivity were demonstrated for specific stocks of Yellowtail Flounder and Winter Flounder, those at the southern extent of the range, while this assessment was conducted at the species level encompassing two or three stocks. The directional effect measure estimates negative effects on both Yellowtail Flounder and Winter Flounder. Similarly, the distribution changes in Alewife and American Shad have been observed during the marine phase only. Natal homing is an important part of the life history of these species [93], so changes in spawning distribution are less likely to occur as predicted by this methodology. The important accomplishment of this assessment is to frame climate vulnerability for a majority of managed fish and invertebrate species in the ecosystem and for a number of unmanaged, but ecologically or commercially important species in the ecosystem.
The assessment was done at the species level. There are pros and cons associated with running a vulnerability assessment at finer (i.e. stock) and coarser (i.e. functional group) levels. A previous assessment in the region was performed at the functional group level [30] and management in many cases is at the stock level (sub-species) [94]. The analysis by functional group (Fig 8, S10 Supporting Information) suggests that while “indicator” species or general functional groups can be used, similar species can have different biological attributes and different overall climate vulnerabilities. A stock specific assessment would be more appropriate to fisheries management, but only in cases where regional species vulnerability is different from stock-specific vulnerability. The choice of number of species (or stocks or functional groups) is dependent on the resources available and the objectives of the assessment.
Our objective here was to provide a broad examination of climate vulnerability for fish and invertebrates on the Northeast U.S. shelf. There are other approaches to estimating species’ vulnerability to climate change. For example, thermal habitat modeling was completed to estimate available future thermal habitat on the Scotian Shelf [15, 95]. While objectives were similar, the methodologies are different enough that the results provide answers to different questions (projected available habitat vs trait based vulnerabilities). Mechanistic climate-population models are another way of investigating climate vulnerability. Models have been developed for a few species (<10 species) but to complete such detailed analyses for the 82 species included here will take several years at least [11, 14, 77]. Finally, Bioclimatic Envelop Modeling has been conducted at the global [19, 20] and regional scale [96]. All of these approaches have value and can contribute to informing managers of the challenges and opportunities that result from climate change. Together, these studies indicate that climate change is going to impact fish and invertebrate species for the foreseeable future.
In addition to addressing scientific questions and identifying important research needs, the results of this assessment can be used by managers in at least four ways. First, the information resulting from the assessment can be used to inform management and regulatory documents, including fishery management plans developed under the Magnuson-Stevens Fishery Conservation and Management Act, Biological Opinions, and Listing Decisions under the Endangered Species Act, and Environmental Impact Statements under the National Environmental Policy Act [97]. Second, the results can be used to guide management actions. The climate vulnerability of 8 species decreases if Stock Status is removed from the analysis: Atlantic Halibut, Winter Flounder, Witch Flounder, Barndoor Skate, Dusky Shark, Porbeagle, Rossette Skate, Sand Tiger, and Thorny Skate. Efforts to decrease fishing mortality on these species might result in an increase in stock size and thus lower overall climate vulnerability. Third, the assessment can contribute to the development of regional Ecosystem-Based Fisheries Management [98], inform spatial management (e.g., management areas for future refuges of vulnerable species), and guide the inclusion of climate variables in single-, multi-species and ecosystem models [99, 100]. Fourth, the vulnerability narratives are useful for identification of species specific research and management needs as they provide in-depth species specific scores as well as a discussion of earlier climate related studies on that species.
We advocate that the Fisheries Climate Vulnerability Assessment should be conducted iteratively. Most fishery stock assessments are conducted iteratively [101] and we recommend conducting the climate vulnerability assessments following the same interval as the IPCC Assessment Reports. Similarly, Integrated Ecosystem Assessments are planned to be iterative [102]. The implementation of the Climate Vulnerability Assessment Methodology was successful (S11 Supporting Information). However, there are improvements that should be considered for the next iteration. First, bottom temperature and improved ocean acidification projections should be included; both of these would require some form of regional downscaling [77] or higher resolution climate models [103]. Second, the ensemble uncertainty in climate change could also be included formally in the assessment (S12 Supporting Information), along with different emission scenarios and potentially time periods. Third, the next iteration should include an update of the species profiles and rescoring of both the climate exposure and sensitivity attributes. If information on species specific plasticity or evolutionary adaptability exist by the next iteration, this information should be included to improve the evaluation of adaptive capacity [33, 104, 105]. Fourth, the next assessment should include experts from a broader background than used here. This assessment included only NMFS employees with some outside observers. One of the main objectives of this effort was to provide the first implementation of NMFS Methodology [34]. This will not be an objective of the next iteration and efforts should be made to include a broad array of fisheries, protected species, and ecosystem stakeholders. Fifth, there are potential links that could be made between Overfishing Vulnerability Assessments [24, 25], Habitat Vulnerability Assessments [106] and Social Vulnerability Assessments [107]. The vulnerability assessment framework provides a powerful tool that can complement other assessment techniques and support the broader implementation of Ecosystem-Based Management and climate change adaptation strategies.
Supporting Information
(CSV)
(CSV)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
Funding for this project was provided by the NOAA NMFS Office of Science and Technology, NOAA NMFS Office of Sustainable Fisheries, NOAA OAR Earth System Laboratory, NOAA NMFS Greater Atlantic Regional Fisheries Office, NOAA NMFS Northeast Fisheries Science Center, and the NOAA Ocean Acidification Program. We thank Antonietta Capotondi, Vince Saba, and Charles Stock for contributing to the description of projected changes in ocean currents in the Northwest Atlantic Ocean. We thank Mike Johnson for contributing to the description of projected sea level rise in the region. We thank Richard Balouskus, Diane Borggaard, Edith Carson, Sarah Laporte, Lynn Lankshear, Jessica Pruden, Rory Saunders, and Tara Trinko Lake for completing species profiles in preparation for the assessment. We thank Kim Hare for formatting references here and in the Supplemental Material and we thank Kris Gamble for help with the figures. Finally, we thank Diane Borggaard and Kimberly Damon-Randall for the encouragement and support throughout the project (before the beginning to the end). We also thank Ken Drinkwater, Jeff Hutchings, and Nick Caputi for their thoughtful and constructive advice during a Council of Independent Experts review of the Climate Vulnerability Assessment Methodology and this Northeast fisheries climate vulnerability assessment. Acknowledgment of the above individuals does not imply their endorsement of this work; the authors have sole responsibility for the content of this contribution. The views expressed herein are those of the authors and do not necessarily reflect the views of NOAA or any of its sub-agencies.
Data Availability
All relevant data is available in the paper and its Supporting Information files.
Funding Statement
Funding for this project was provided by the National Oceanic and Atmospheric Administration (NOAA) NMFS Office of Science and Technology, NOAA NMFS Office of Sustainable Fisheries, NOAA OAR Earth System Laboratory, NOAA NMFS Greater Atlantic Regional Fisheries Office, NOAA NMFS Northeast Fisheries Science Center, and the NOAA Ocean Acidification Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Baumgartner TR, Soutar A, Ferreira-Bartrina V. Reconstruction of the history of Pacific sardine and northern anchovy populations over the past two millennia from sediments of the Santa Barbara Basin, California. CalCOFI Rep. 1992; 33: 24–40. [Google Scholar]
- 2.Perry AL, Low PJ, Ellis JR, Reynolds JD. Climate change and distribution shifts in marine fishes. Science. 2005; 308(5730): 1912–1915. 10.1126/science.1111322 [DOI] [PubMed] [Google Scholar]
- 3.Mueter FJ, Litzow MA. Sea ice retreat alters the biogeography of the Bering Sea continental shelf. Ecol Appl. 2008; 18(2): 309–320. 10.1890/07-0564.1 [DOI] [PubMed] [Google Scholar]
- 4.Hare JA, Able KW. Mechanistic links between climate and fisheries along the east coast of the United States: explaining population outbursts of Atlantic croaker (Micropogonias undulatus). Fish Oceanogr. 2007; 16(1): 31–45. 10.1111/j.1365-2419.2006.00407.x [DOI] [Google Scholar]
- 5.Petitgas P, Alheit J, Peck MA, Raab K, Irigoien X, Huret M, et al. 2012. Anchovy population expansion in the North Sea. Mar Ecol Prog Ser. 2012; 444: 1–13. 10.3354/meps09451 [DOI] [Google Scholar]
- 6.Pinsky ML, Fogarty M. Lagged social-ecological responses to climate and range shifts in fisheries. Climatic change. 2012; 115(3–4): 883–891. 10.1007/s10584-012-0599-x [DOI] [Google Scholar]
- 7.Gamito R, Teixeira CM, Costa MJ, Cabral HN. Are regional fisheries’ catches changing with climate?. Fish Res. 2015; 161: 207–216. 10.1016/j.fishres.2014.07.014 [DOI] [Google Scholar]
- 8.Bell RJ, Hare JA, Manderson JP, Richardson DE. Externally driven changes in the abundance of summer and winter flounder. ICES J. Mar. Sci. 2014; 71(9): 2416–2428. 10.1093/icesjms/fsu069 [DOI] [Google Scholar]
- 9.Perry RI, Cury P, Brander K, Jennings S, Möllmann C, Planque B. Sensitivity of marine systems to climate and fishing: concepts, issues and management responses. J Mar Syst. 2010; 79(3): 427–435. 10.1016/j.jmarsys.2008.12.017 [DOI] [Google Scholar]
- 10.Hollowed AB, Barange M, Beamish RJ, Brander K, Cochrane K, Drinkwater K, et al. Projected impacts of climate change on marine fish and fisheries. ICES J Mar Sci. 2013; 70(5): 1023–1037. 10.1093/icesjms/fst081 [DOI] [Google Scholar]
- 11.Fogarty M, Incze L, Hayhoe K, Mountain D, Manning J. Potential climate change impacts on Atlantic cod (Gadus morhua) off the northeastern USA. Mitig Adapt Strat Glob Change. 2008; 13(5–6): 453–466. 10.1007/s11027-007-9131-4 [DOI] [Google Scholar]
- 12.Hollowed AB, Bond NA, Wilderbuer TK, Stockhausen WT, A'mar ZT, Beamish RJ, et al. A framework for modelling fish and shellfish responses to future climate change. ICES J Mar Sci. 2009; 66(7): 1584–1594. 10.1093/icesjms/fsp057 [DOI] [Google Scholar]
- 13.Lehodey P, Senina I, Sibert J, Bopp L, Calmettes B, Hampton J, et al. Preliminary forecasts of Pacific bigeye tuna population trends under the A2 IPCC scenario. Prog Oceanogr. 2010; 86: 302–315. 10.1016/j.pocean.2010.04.021 [DOI] [Google Scholar]
- 14.Hare JA, Alexander MA, Fogarty MJ, Williams EH, & Scott JD. Forecasting the dynamics of a coastal fishery species using a coupled climate-population model. Ecol Appl. 2010; 20(2): 452–464. 10.1890/08-1863.1 [DOI] [PubMed] [Google Scholar]
- 15.Shackell NL, Ricard D, Stortini CH. Thermal habitat index of many Northwest Atlantic temperate species stays neutral under warming projected for 2030 but changes radically by 2060. PloS ONE. 2014; 9(3): e90662 10.1371/journal.pone.0090662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooley SR, Rheuban JE, Hart DR, Luu V, Glover DM, Hare JA, et al. An Integrated Assessment Model for Helping the United States Sea Scallop (Placopecten magellanicus) Fishery Plan Ahead for Ocean Acidification and Warming. PLoS ONE. 2015; 10(5): e0124145 10.1371/journal.pone.0124145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stock CA, Alexander MA, Bond NA, Brander KM, Cheung WW, Curchitser EN, et al. On the use of IPCC-class models to assess the impact of climate on living marine resources. Prog Oceanogr. 2011; 88(1): 1–27. 10.1016/j.pocean.2010.09.001 [DOI] [Google Scholar]
- 18.Barbier EB. Progress and challenges in valuing coastal and marine ecosystem services. Rev Environ Econ Pol. 2012; 6(1): 1–19. 10.1093/reep/rer017 [DOI] [Google Scholar]
- 19.Cheung WW, Lam VW, Sarmiento JL, Kearney K, Watson R, Pauly D. Projecting global marine biodiversity impacts under climate change scenarios. Fish Fisher. 2009; 10(3), 235–251. 10.1111/j.1467-2979.2008.00315.x [DOI] [Google Scholar]
- 20.Jones MC, Cheung WW. Multi-model ensemble projections of climate change effects on global marine biodiversity. ICES J Mar Sci. 2015; 72(3): 741–752. 10.1093/icesjms/fsu172 [DOI] [Google Scholar]
- 21.Foden WB, Butchart SH, Stuart SN, Vié JC Akçakaya HR, Angulo A, et al. Identifying the world's most climate change vulnerable species: a systematic trait-based assessment of all birds, amphibians and corals. PLoS ONE. 2013; 8(6): e65427 10.1371/journal.pone.0065427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glick P, Stein BA, Edelson NA, editors. Scanning the Conservation Horizon: A Guide to Climate Change Vulnerability Assessment. 2011. National Wildlife Federation, Washington, D.C. Available: http://www.nwf.org/vulnerabilityguide [Google Scholar]
- 23.Williams SE, Shoo LP, Isaac JL, Hoffmann AA, Langham G. Towards an integrated framework for assessing the vulnerability of species to climate change. PLoS biology. 2008; 6(12): e325 10.1371/journal.pbio.0060325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patrick WS, Spencer P, Link J, Cope J, Field J, Kobayashi D, et al. Using productivity and susceptibility indices to assess the vulnerability of United States fish stocks to overfishing. Fish Bull. 2010; 108(3): 305–322. Available: http://fishbull.noaa.gov/1083/patrick.pdf [Google Scholar]
- 25.Hobday AJ, Smith ADM, Stobutzki IC, Bulman C, Daley R, Dambacher, et al. Ecological risk assessment for the effects of fishing. Fish Res. 2011; 108(2): 372–384. 10.1016/j.fishres.2011.01.013 [DOI] [Google Scholar]
- 26.Johnson JE, Welch DJ. Marine fisheries management in a changing climate: a review of vulnerability and future options. Rev Fish Sci. 2009; 18(1): 106–124. 10.1080/10641260903434557 [DOI] [Google Scholar]
- 27.Chin A, Kyne PM, Walker TI, McAuley RB. An integrated risk assessment for climate change: analysing the vulnerability of sharks and rays on Australia's Great Barrier Reef. Glob Chang Biol. 2010; 16(7): 1936–1953. 10.1111/j.1365-2486.2009.02128.x [DOI] [Google Scholar]
- 28.Doubleday ZA, Clarke SM, Li X, Pecl GT, Ward TM, Battaglene S, et al. (2013). Assessing the risk of climate change to aquaculture: a case study from south-east Australia. Aquacult Environ Interact. 2013; 3(2): 163–175. 10.3354/aei00058 [DOI] [Google Scholar]
- 29.Pecl GT, Ward TM, Doubleday ZA, Clarke S, Day J, Dixon C, et al. Rapid assessment of fisheries species sensitivity to climate change. Climatic Change, 2014; 127(3–4): 505–520. 10.1007/s10584-014-1284-z [DOI] [Google Scholar]
- 30.Gaichas SK, Link JS, Hare JA. A risk-based approach to evaluating northeast US fish community vulnerability to climate change. ICES J Mar Sci. 2014; 71(8): 2323–2342. 10.1093/icesjms/fsu048 [DOI] [Google Scholar]
- 31.Mathis JT, Cooley SR, Lucey N, Colt S, Ekstrom J, Hurst T, et al. Ocean acidification risk assessment for Alaska’s fishery sector. Progr Oceanogr. 2014; 136: 71–91. 10.1016/j.pocean.2014.07.001 [DOI] [Google Scholar]
- 32.Garcia RA, Araújo MB, Burgess ND, Foden WB, Gutsche A, Rahbek C, et al. Matching species traits to projected threats and opportunities from climate change. J Biogeogr. 2014; 41(4), 724–735. 10.1111/jbi.12257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beever EA, O'Leary J, Mengelt C, West JM, Julius S, Green N, et al. Improving conservation outcomes with a new paradigm for understanding species’ fundamental and realized adaptive capacity. Conservation Letters. 2015: 10.1111/conl.12190 [DOI] [Google Scholar]
- 34.Morrison W, Nelson M, Howard J, Teeters E, Hare JA, Griffis R, et al. in press. Methodology for assessing the vulnerability of fish stocks to a changing climate. NOAA Technical Memorandum 2015; NMFS-OSF-3:1–48. Available: https://www.st.nmfs.noaa.gov/Assets/ecosystems/climate/documents/TM%20OSF3.pdf
- 35.Pörtner HO. Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp Biochem Physiol A Mol Integr Physiol. 2002; 132(4): 739–61. hdl:10013/epic.15426.d001 [DOI] [PubMed] [Google Scholar]
- 36.Solomon A, Goddard L, Kumar A, Carton J, Deser C, Fukumori I. Distinguishing the roles of natural and anthropogenically forced decadal climate variability: implications for prediction. Bull Amer Meteorol Soc. 2011; 92(2): 141–156. 10.1175/2010BAMS2962.1 [DOI] [Google Scholar]
- 37.National Marine Fisheries Service. 2014a. Commercial fisheries statistics. Available: http://www.st.nmfs.noaa.gov/commercial-fisheries/commercial-landings/annual-landings/index
- 38.Lovell S, Steinback S, Hilger J. The Economic Contribution of Marine Angler Expenditures in the United States, 2011. U.S. Dep. Commerce, NOAA Tech. Memo. 2013 NMFS-F/SPO-134, 188 p. Available: http://www.st.nmfs.noaa.gov/Assets/economics/publications/AnglerExpenditureReport/2011/pdf/The%20Economic%20Contribution%20of%20Marine%20Angler%20Expenditures%20in%20the%20United%20States%202011.pdf
- 39.Melillo JM, Richmond TC, Yohe GW, editors. 2014: Climate Change Impacts in the United States: The Third National Climate Assessment. U.S. Global Change Research Program, 841 pp. 10.7930/J0Z31WJ2 [DOI] [Google Scholar]
- 40.Weinberg JR. Bathymetric shift in the distribution of Atlantic surfclams: response to warmer ocean temperature. ICES J Mar Sci. 2005; 62(7): 1444–1453. 10.1016/j.icesjms.2005.04.020 [DOI] [Google Scholar]
- 41.Nye JA, Link JS, Hare JA, Overholtz WJ. Changing spatial distribution of fish stocks in relation to climate and population size on the Northeast United States continental shelf. Mar Ecol Prog Ser. 2009; 393: 111–129. 10.3354/meps08220 [DOI] [Google Scholar]
- 42.Pinsky ML, Worm B, Fogarty MJ, Sarmiento JL, Levin SA. Marine taxa track local climate velocities. Science. 2013; 341(6151): 1239–1242. 10.1126/science.1239352 [DOI] [PubMed] [Google Scholar]
- 43.Lynch PD, Nye JA, Hare JA, Stock CA, Alexander MA, Scott JD, et al. (2015). Projected ocean warming creates a conservation challenge for river herring populations. ICES J Mar Sci. 2015; 72(2): 374–387. 10.1093/icesjms/fsu134 [DOI] [Google Scholar]
- 44.Northeast Fisheries Science Center. 54th Northeast Regional Stock Assessment Workshop (54th SAW) Assessment Report. US Dept Commerce, Northeast Fish Sci Cent Ref Doc. 2012.; 12–18: 600 p. Available: http://www.asmfc.org/uploads/file/crd1218.pdf
- 45.Federal Register. Endangered and Threatened Wildlife and Plants; Endangered Species Act Listing Determination for Alewife and Blueback Herring. Vol. 78, No. 155 48944–48994 http://www.gpo.gov/fdsys/pkg/FR-2013-08-12/html/2013-19380.htm
- 46.Able KW, Fahay MP. Ecology of estuarine fishes: temperate waters of the western North Atlantic Baltimore: Johns Hopkins University Press; 2010. [Google Scholar]
- 47.National Marine Fisheries Service. Endangered and Threatened Marine Species under NMFS' Jurisdiction. Available: http://www.nmfs.noaa.gov/pr/species/esa/listed.htm#fish
- 48.National Marine Fisheries Service. NMFS Proactive Conservation Program: Species of Concern. Available: http://www.nmfs.noaa.gov/pr/species/concern/
- 49.Mid-Atlantic Fishery Management Council (MAFMC). Protecting Unmanaged Forage Species. Available: http://www.mafmc.org/actions/unmanaged-forage
- 50.Pörtner HO, Peck MA. Climate change effects on fishes and fisheries: towards a cause-and-effect understanding. J Fish Biol. 2010; 77: 1745–1779. 10.1111/j.1095-8649.2010.02783.x [DOI] [PubMed] [Google Scholar]
- 51.Hettler WF. Influence of temperature and salinity on routine metabolic rate and growth of young Atlantic menhaden. J Fish Biol. 1976; 8: 55–65. 10.1111/j.1095-8649.1976.tb03907.x [DOI] [Google Scholar]
- 52.Ji R, Davis CS, Chen C, Townsend DW, Mountain DG, Beardsley RC. Influence of ocean freshening on shelf phytoplankton dynamics. Geophys Res Lett. 2007; 34(24). 10.1029/2007GL032010 [DOI] [Google Scholar]
- 53.Pilgrim JM, Fang X, Stefan HG. Stream temperature correlations with air temperatures in Minnesota: implications for climate warming. J Am Water Resour Assoc. 1998; 34(5): 1109–1121. 10.1111/j.1752-1688.1998.tb04158.x [DOI] [Google Scholar]
- 54.Crecco VA, Savoy TF. Effects of biotic and abiotic factors on growth and relative survival of young American shad, Alosa sapidissima, in the Connecticut River. Can J Fish Aquat Sci. 1985; 42(10): 1640–1648. 10.1139/f85-205 [DOI] [Google Scholar]
- 55.Walsh H.J, Settle LR, Peters DS. Early life history of blueback herring and alewife in the lower Roanoke River, North Carolina. Trans Am Fish Soc. 2005; 134(4): 910–926. 10.1577/T04-060.1 [DOI] [Google Scholar]
- 56.Munday PL, Dixson DL, Donelson JM, Jones GP, Pratchett MS, Devitsina GV, et al. Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc Nat Acad Sci. 2009; 106(6): 1848–1852. 10.1073/pnas.0809996106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Talmage SC. Gobler CJ. Effects of past, present, and future ocean carbon dioxide concentrations on the growth and survival of larval shellfish. Proc Nat Acad Sci. 2010; 107(40): 17246–17251. 10.1073/pnas.0913804107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pineda J, Hare JA, Sponaungle S. Larval transport and dispersal in the coastal ocean and consequences for population connectivity. Oceanogr. 2007; 20(3): 22–39. 10.5670/oceanog.2007.27 [DOI] [Google Scholar]
- 59.Craft C, Clough J, Ehman J, Joye S, Park R, Pennings S, et al. Forecasting the effects of accelerated sea-level rise on tidal marsh ecosystem services. Front Ecol Environ. 2008; 7(2): 73–78. 10.1890/070219 [DOI] [Google Scholar]
- 60.Hoegh-Guldberg O, Bruno JF. The impact of climate change on the world’s marine ecosystems. Science. 2010; 328(5985): 1523–1528. 10.1126/science.1189930 [DOI] [PubMed] [Google Scholar]
- 61.Titus JG, Anderson KE, Cahoon DR, Gesch DB, Gill SK, Gutierrez BT, et al. Coastal sensitivity to sea-level rise: a focus on the Mid-Atlantic Region Synthesis and Assessment Product 4.1; Report by the U.S. Climate Change Science Program and the Subcommittee on Global Change Research. 2009. 298 pp. U.S. Climate Change Science Program, Washington, DC: http://permanent.access.gpo.gov/LPS110750/LPS110750/downloads.climatescience.gov/sap/sap4-1/sap4-1-final-report-all.pdf [Google Scholar]
- 62.Atlantic States Marine Fisheries Commission (ASMFC). Atlantic Croaker 2010 Benchmark Stock Assessment. 2010. Available: http://www.asmfc.org/uploads/file//5282798aatlanticCroaker2010BenchmarkStockAssessment.pdf
- 63.Northeast Fisheries Science Center. Northeast Regional Stock Assessment Workshop (SAW) 2014. Available: http://www.nefsc.noaa.gov/saw/
- 64.Northeast Fisheries Science Center. EFH Source Documents: Life History and Habitat Characteristics. 2014. Available: http://www.nefsc.noaa.gov/nefsc/habitat/efh/
- 65.Murdy EO, Birdsong RS, Musick JA. Fishes of Chesapeake Bay (p. 324). Washington, DC: Smithsonian Institution Press, 1997. [Google Scholar]
- 66.Collette BB and Klein-MacPhee G. editors. Bigelow and Schroeder’s Fishes of the Gulf of Maine. Washington, D.C.: Smithsonian Institution Press; 2002. [Google Scholar]
- 67.Flato G, Marotzke J, Abiodun B, Braconnot P, Chou SC, Collins W, et al. Evaluation of Climate Models. In: Climate Change 2013: The Physical Science Basis Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, et al. (eds.)]. 2013. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA: https://www.ipcc.ch/pdf/assessment-report/ar5/wg1/WG1AR5_Chapter09_FINAL.pdf [Google Scholar]
- 68.Riahi K, Rao S, Krey V, Cho C, Chirkov V, Fischer G, et al. RCP 8.5—A scenario of comparatively high greenhouse gas emissions. Climatic Change. 2011; 109(1–2): 33–57. 10.1007/s10584-011-0149-y [DOI] [Google Scholar]
- 69.Van Vuuren DP, Edmonds J, Kainuma M, Riahi K, Thomson A, Hibbard K, et al. The representative concentration pathways: an overview. Climatic Change. 2011; 109: 5–31. 10.1007/s10584-011-0148-z [DOI] [Google Scholar]
- 70.Earth Systems Research Laboratory. NOAA’s Ocean Climate Change Web Portal. 2014. Available: http://www.esrl.noaa.gov/psd/ipcc/ocn/
- 71.Tewksbury JJ, Huey RB, Deutsch CA. Putting the heat on tropical animals. Science. 2008; 320: 1296–1297. 10.1126/science.1159328 [DOI] [PubMed] [Google Scholar]
- 72.Sunday JM, Bates AE, Dulvy NK. Global analysis of thermal tolerance and latitude in ectotherms. Proc Biol Sci. 2011; 278(1713): 1823–1830. 10.1098/rspb.2010.1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ocean Biogeographic Information System. Available: http://www.iobis.org/
- 74.Sunday JM, Pecl GT, Frusher S, Hobday AJ, Hill N, Holbrook NJ, et al. Species traits and climate velocity explain geographic range shifts in an ocean‐warming hotspot. Ecol Lett. 2015; 18(9): 944–953. 10.1111/ele.12474 [DOI] [PubMed] [Google Scholar]
- 75.Walsh HJ, Richadson DE, Marancik KE, Hare JA. Long-term changes in the distributions of larval and adult fish on the northeast U.S. shelf. PLoS ONE. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bell RJ, Richardson DE, Hare JA, Lynch PD, Fratantoni PS. Disentangling the effects of climate, abundance, and size on the distribution of marine fish: an example based on four stocks from the Northeast US shelf. ICES J. Mar. Sci. 2014; 72(5): 1311–1322. 10.1093/icesjms/fsu217 [DOI] [Google Scholar]
- 77.Hare JA., Manderson JP, Nye JA, Alexander MA, Auster PJ, Borggaard DL, et al. Cusk (Brosme brosme) and climate change: assessing the threat to a candidate marine fish species under the US Endangered Species Act. ICES J Mar Sci. 2012; 69(10): 1753–1768. 10.1093/icesjms/fss160 [DOI] [Google Scholar]
- 78.Pershing AJ, Alexander MA, Hernandez CM, Kerr LA, Le Bris A, Mills KE, et al. Slow adaptation in the face of rapid warming leads to collapse of the Gulf of Maine cod fishery. Science, 2015; 350(6262): 809–812. 10.1126/science.aac9819 [DOI] [PubMed] [Google Scholar]
- 79.Link JS, Nye JA, Hare JA. Guidelines for incorporating fish distribution shifts into a fisheries management context. Fish Fish. 2011; 12(4): 461–469. 10.1111/j.1467-2979.2010.00398.x [DOI] [Google Scholar]
- 80.Friedland KD, Hare JA. Long-term trends and regime shifts in sea surface temperature on the continental shelf of the northeast United States. Cont Shelf Res. 2007; 27(18): 2313–2328. 10.1016/j.csr.2007.06.001 [DOI] [Google Scholar]
- 81.Mills KE, Pershing AJ, Brown CJ, Chen Y, Chiang FS, Holland DS, et al. Fisheries management in a changing climate: lessons from the 2012 ocean heat wave in the Northwest Atlantic. Oceanography 2013; 26(2): 191–195. 10.5670/oceanog.2013.27 [DOI] [Google Scholar]
- 82.Brown JH, Gillooly JF, Allen AP, Savage VM, West, GB. Toward a metabolic theory of ecology. Ecology. 2004; 85(7), 1771–1789. 10.1890/03-9000 [DOI] [Google Scholar]
- 83.Helmuth B, Mieszkowska N, Moore P, Hawkins SJ. Living on the edge of two changing worlds: forecasting the responses of rocky intertidal ecosystems to climate change. Annual Review of Ecology, Evolution, and Systematics, 2006; 37: 373–404. 10.1146/annurev.ecolsys.37.091305.110149 [DOI] [Google Scholar]
- 84.Gaston KJ, Chown SL, Calosi P, Bernardo J, Bilton DT, Clarke A, et al. Macrophysiology: a conceptual reunification. Am Nat. 2009; 174(5): 595–612. 10.1086/605982 [DOI] [PubMed] [Google Scholar]
- 85.Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo L, Singh GS, et al. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob Change Biol. 2013; 19(6): 1884–1896. 10.1111/gcb.12179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chambers RC, Candelmo AC, Habeck EA, Poach ME, Wieczorek D, Cooper KR, et al. Effects of elevated CO2 in the early life stages of summer flounder, Paralichthys dentatus, and potential consequences of ocean acidification. Biogeosciences. 2014; 11, 1613–1626. 10.5194/bg-11-1613-2014 [DOI] [Google Scholar]
- 87.Signorini SR, Mannino A, Najjar RG, Friedrichs MA, Cai WJ, Salisbury J. et al. Surface ocean pCO2 seasonality and sea‐air CO2 flux estimates for the North American east coast. J Geophys Res Oceans, 2013; 118(10): 5439–5460. 10.1002/jgrc.20369 [DOI] [Google Scholar]
- 88.Strayer DL, Cole JJ, Findlay SE, Fischer DT, Gephart JA, Malcom HM, et al. Decadal-scale change in a large-river ecosystem. BioScience, 2014; 64(6): 496–510. 10.1093/biosci/biu061 [DOI] [Google Scholar]
- 89.Hahn ME. Mechanistic research in aquatic toxicology: perspectives and future directions. Aquat Toxicol. 2011; 105(3): 67–71. 10.1016/j.aquatox.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mahrosh U, Kleiven M, Meland S, Rosseland BO, Salbu B, & Teien HC. Toxicity of road deicing salt (NaCl) and copper (Cu) to fertilization and early developmental stages of Atlantic salmon (Salmo salar). J Hazard Mater. 2014; 280: 331–339. 10.1016/j.jhazmat.2014.07.076 [DOI] [PubMed] [Google Scholar]
- 91.Hall CJ, Jordaan A, Frisk MG. The historic influence of dams on diadromous fish habitat with a focus on river herring and hydrologic longitudinal connectivity. Landsc Ecol. 2011; 26(1): 95–107. 10.1007/s10980-010-9539-1 [DOI] [Google Scholar]
- 92.Veilleux HD, Ryu T, Donelson JM, Herwerden L, Seridi L, Ghosheh Y., et al. Molecular processes of transgenerational acclimation to a warming ocean. Nat Clim Chang. 2015. 10.1038/nclimate2724 [DOI] [Google Scholar]
- 93.Walther BD, Thorrold SR, Olney JE. Geochemical signatures in otoliths record natal origins of American shad. Trans Am Fish Soc. 2008; 137(1): 57–69. 10.1577/T07-029.1 [DOI] [Google Scholar]
- 94.Northeast Fisheries Science Center. Assessment of 19 Northeast Groundfish Stocks through 2007: Report of the 3rd Groundfish Assessment Review Meeting (GARM III), Northeast Fisheries Science Center, Woods Hole, Massachusetts, August 4–8, 2008. US Dept Commerce, NOAA FIsheries, Northeast Fish Sci Cent Ref Doc. 08–15; 884 p + xvii. Available: http://www.nefsc.noaa.gov/publications/crd/crd0815/
- 95.Stortini CH, Shackell NL, Tyedmers P, Beazley K. Assessing marine species vulnerability to projected warming on the Scotian Shelf, Canada. ICES J Mar Sci. 2015; 72(6): 1731–1743. 10.1093/icesjms/fsv022 [DOI] [Google Scholar]
- 96.Cheung WW, Brodeur RD, Okey TA, Pauly D. Projecting future changes in distributions of pelagic fish species of Northeast Pacific shelf seas. Prog Oceanogr. 2015; 130: 19–31 [Google Scholar]
- 97.Seney EE, Rowland MJ, Lowery RA, Griffis RB, McClure MM. Climate Change, Marine Environments, and the U.S. Endangered Species Act. Conserv Biol. 2013; 27(6): 1138–1146. 10.1111/cobi.12167 [DOI] [PubMed] [Google Scholar]
- 98.Link J. Ecosystem-based fisheries management: confronting tradeoffs Cambridge University Press, 2010. [Google Scholar]
- 99.Nye JA, Gamble RJ, Link JS. The relative impact of warming and removing top predators on the Northeast US large marine biotic community. Ecol Modell. 2013; 264: 157–168. 10.1016/j.ecolmodel.2012.08.019 [DOI] [Google Scholar]
- 100.Busch DS, Harvey CJ, & McElhany P. Potential impacts of ocean acidification on the Puget Sound food web. ICES J Mar Sci. 2013; 70(4): 823–833. 10.1093/icesjms/fst061 [DOI] [Google Scholar]
- 101.Methot RD Jr.. Stock assessment: operational models in support of fisheries management In The Future of Fisheries Science in North America (pp. 137–165). Springer; Netherlands, 2009. [Google Scholar]
- 102.Levin PS, Fogarty MJ, Murawski SA, Fluharty D. Integrated ecosystem assessments: developing the scientific basis for ecosystem-based management of the ocean. PLoS Biology, 2009; 7(1), e1000014 10.1371/journal.pbio.1000014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Saba V, et al. Enhanced warming of the Northwest Atlantic Ocean under climate change. In review
- 104.Somero GN. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J Exp Biol. 2010; 213(6): 912–920. 10.1242/jeb.037473 [DOI] [PubMed] [Google Scholar]
- 105.Hoffmann AA, Sgrò CM. Climate change and evolutionary adaptation. Nature, 2011; 470(7335): 479–485. 10.1038/nature09670 [DOI] [PubMed] [Google Scholar]
- 106.National Marine Fisheries Service. Marine fisheries habitat assessment improvement plan. Report of the National Marine Fisheries Service Habitat Assessment Improvement Plan Team. 2010. U.S. Dep. Commerce, NOAA Tech. Memo. NMFS-F/SPO-108, Available: www.st.nmfs.noaa.gov/st4/documents/habitatAssessmentImprovementPlan_052110.pdf
- 107.Jepson M, Colburn LL. Development of social indicators of fishing community vulnerability and resilience in the US Southeast and Northeast regions. 2013. NOAA Technical Memorandum NMFS-F/SPO-129 (US Dept Commerce, 2013). Available: http://sero.nmfs.noaa.gov/sustainable_fisheries/social/documents/pdfs/communities/2013/vulnerability_resilience_social_indicators.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(CSV)
(CSV)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data is available in the paper and its Supporting Information files.