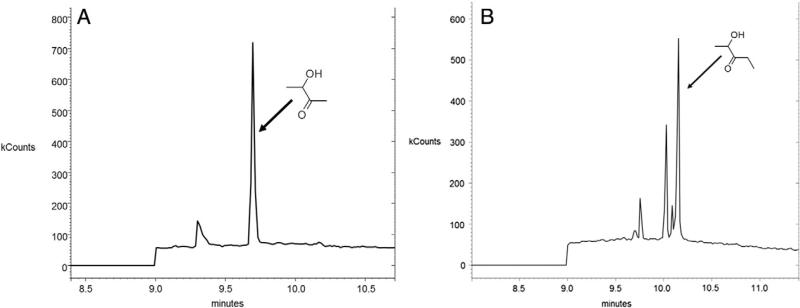
Fig. 10.
Identification of metabolites of diacetyl (acetoin; A) and 2,3-pentanedione (2-hydroxy-3-pentanone; B) in basolateral chamber samples following exposure of NHBEs to 25 ppm diacetyl or 2,3-pentanedione. The arrows point to the peaks identified as acetoin (A) or 2-hydroxy-3-pentanone (B). The diacetyl and 2,3-pentanedione peaks are not shown. The identity of the other peaks is not known; they could be impurities or other metabolites of the flavorings.