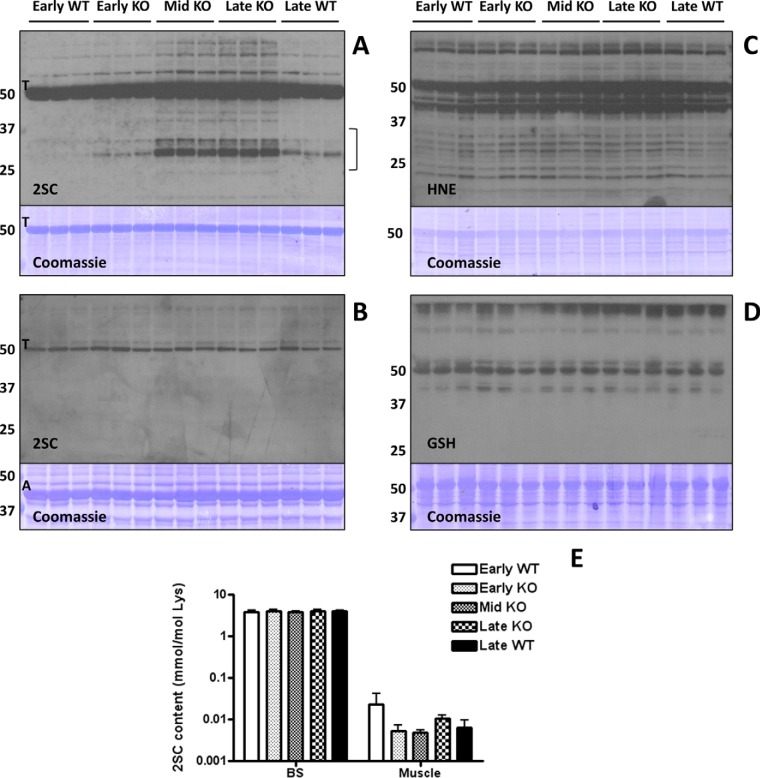
Fig. 1.
Protein succination is increased in the brainstem (BS) of Ndufs4 KO mice. A, Total cell lysates (30 μg protein) from BS of early (3 weeks old), middle (Mid, 6 weeks old) and late (9 weeks old) WT and Ndufs4 KO mice were separated by SDS/PAGE, and succinated proteins were detected using a polyclonal anti-2SC antibody (2SC panel), as described under “Experimental Procedures.” Protein succination in the BS of KO mice increased in association with the age of the mice and progression of disease versus WT controls. Succination was detected on a range of proteins and is especially prominent for proteins of ∼70–80 and ∼25–35 kDa (2SC panel). Note that intense tubulin (T) succination is present in all the lanes, and seems to increase with age in the BS of Ndufs4 KO mice. The bracket denotes the area circled in Fig. 3B. B, Succination of proteins is unchanged in skeletal muscle of Ndufs4 KO mice. Note that even when actin (A) is much more abundant (Coomassie panel), tubulin (T) is the primary succinated protein, confirming the selectivity of the modification. The identity of the tubulin band was confirmed in the BS by LC-MS/MS. C, Protein modification by the reactive lipid peroxidation product 4-hydroxy-2-nonenal (HNE) and (D) glutathione (GSH) is unchanged in the BS of Ndufs4 KO mice. E, The total 2SC content of BS and muscle was determined by GC-MS/MS after acid hydrolysis of the protein. Note that 2SC content (expressed as mmol/mol lysine) is much higher in BS than in muscle. Results are presented as mean ± S.E. No significant differences between ages and genotypes were observed. In all the panels, triplicate samples were used for each age and genotype. In A, B, C, and D molecular masses of marker proteins are indicated on the left-hand side.