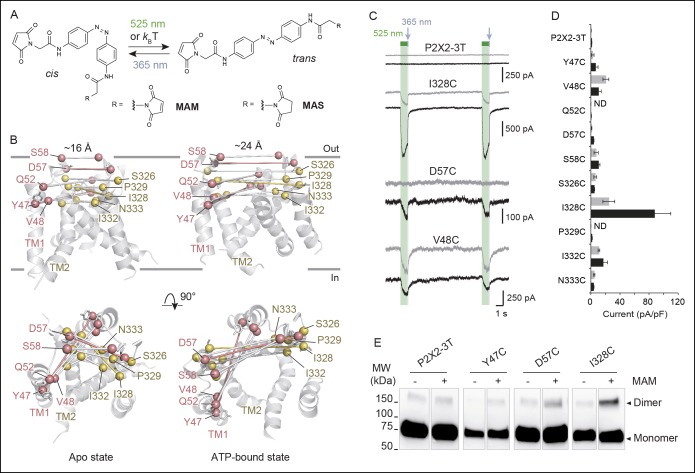
Figure 1. Lateral expansion between TM1 and TM2 helices drives channel opening.
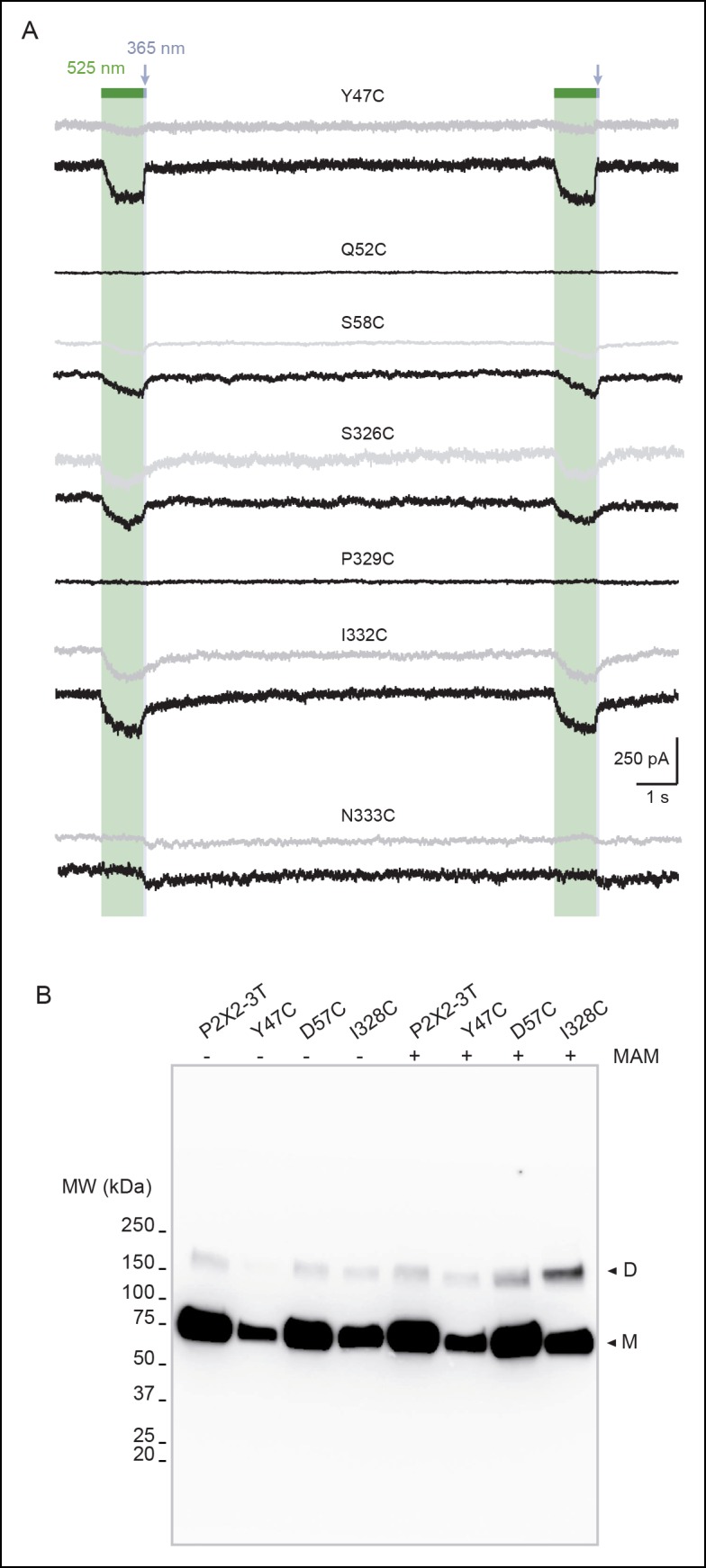
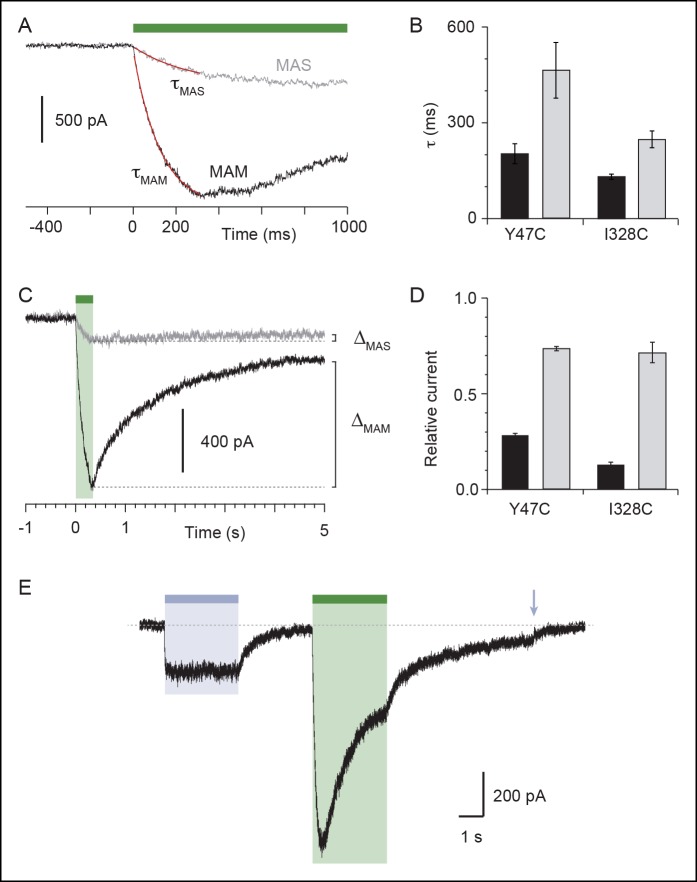
(A) Chemical structures of MAM and MAS in the cis and trans states. (B) Cartoon representation of the TMD of a P2X2 homology model viewed parallel (upper) and perpendicular (lower) to the membrane plane in an apo (left) and ATP-bound state (right). Cβ atoms of residues selected for cysteine substitutions are shown as red and yellow spheres in TM1 and TM2 helices, respectively. Indicated values are the average distances separating pairwise β-atoms from two adjacent subunits (grey bridges). Highlighted bridges indicate actual MAM cross-linking. (C) Whole-cell currents recorded during and after illumination at 525 nm (green bars, 1 s) and 365 nm (violet arrows, 80 ms) in HEK cells expressing the P2X2-3T receptor or the indicated cysteine-substituted mutants after treatment with MAM (black traces) or MAS (gray traces). Just before recordings, cells were irradiated for 85 ms with a light pulse of 365 nm. (D) Screening for all constructs showing light-gated currents following MAM (filled bars) or MAS (gray bars) treatment. All light-gated mutants were activated at 525 nm and inactivated at 365 nm, except for N333C, which responded in the opposite sense to these wavelengths. ND stands for not determined (n = 4–5 cells; mean ± s.e.m.). (E) Western blot analysis of cell-surface cross-linking of the indicated P2X2-3T constructs expressed in TSA-201 cells after treatment (+) or without treatment (-) with MAM. Monomer and dimer are indicated. Uncut gel image is shown in Figure 1—figure supplement 2B. MAM: 4,4´-bis(maleimido-glycine)azobenzene; MAS: 4-(maleimido-glycine)-4'-(succimido-glycine)azobenzene; MW: Molecular weight; TMD: Transmembrane domain.
DOI: http://dx.doi.org/10.7554/eLife.11050.003
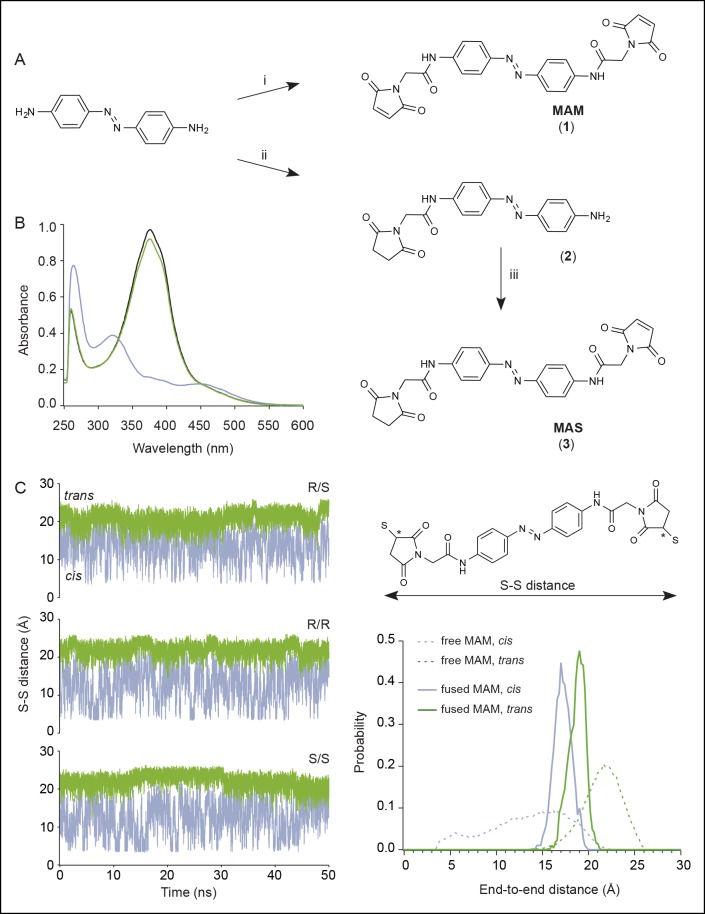
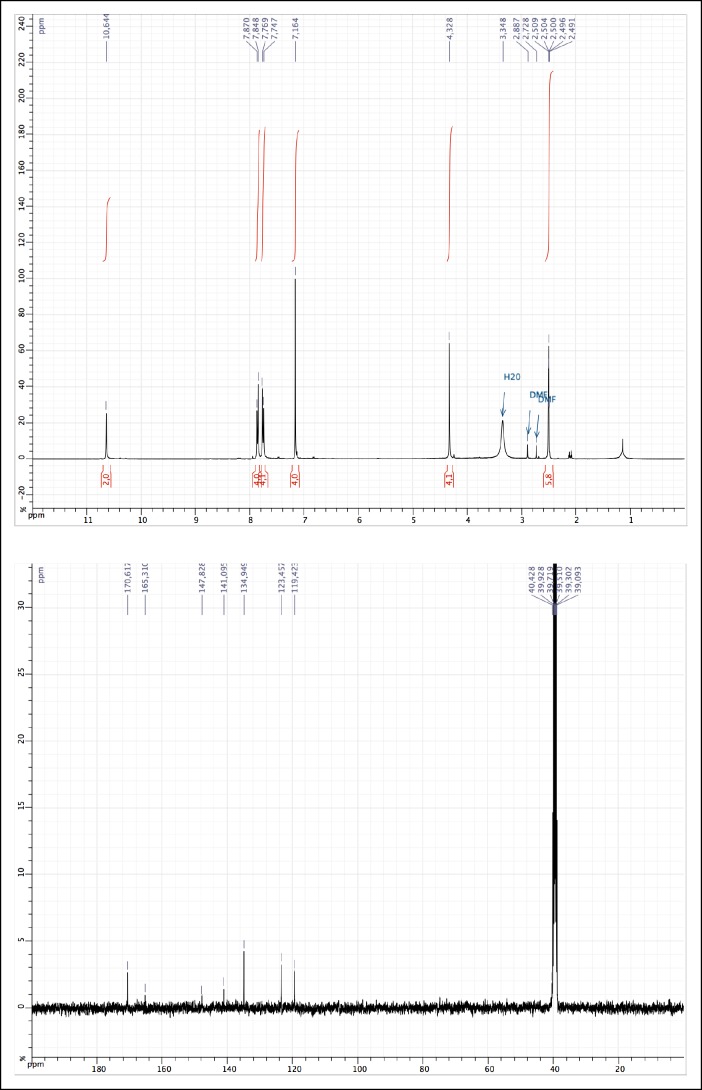
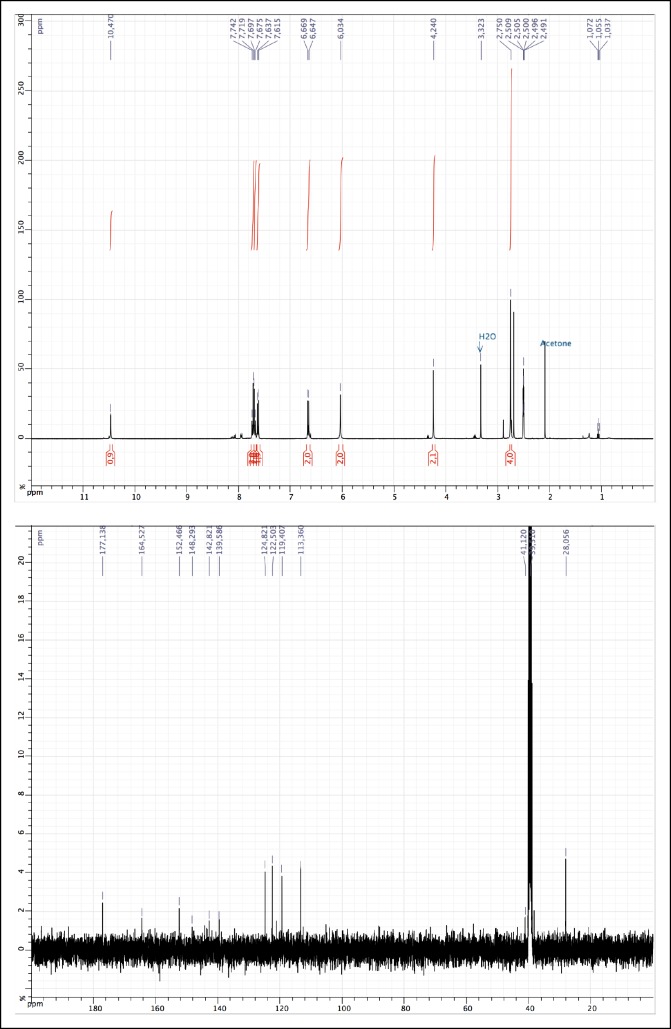
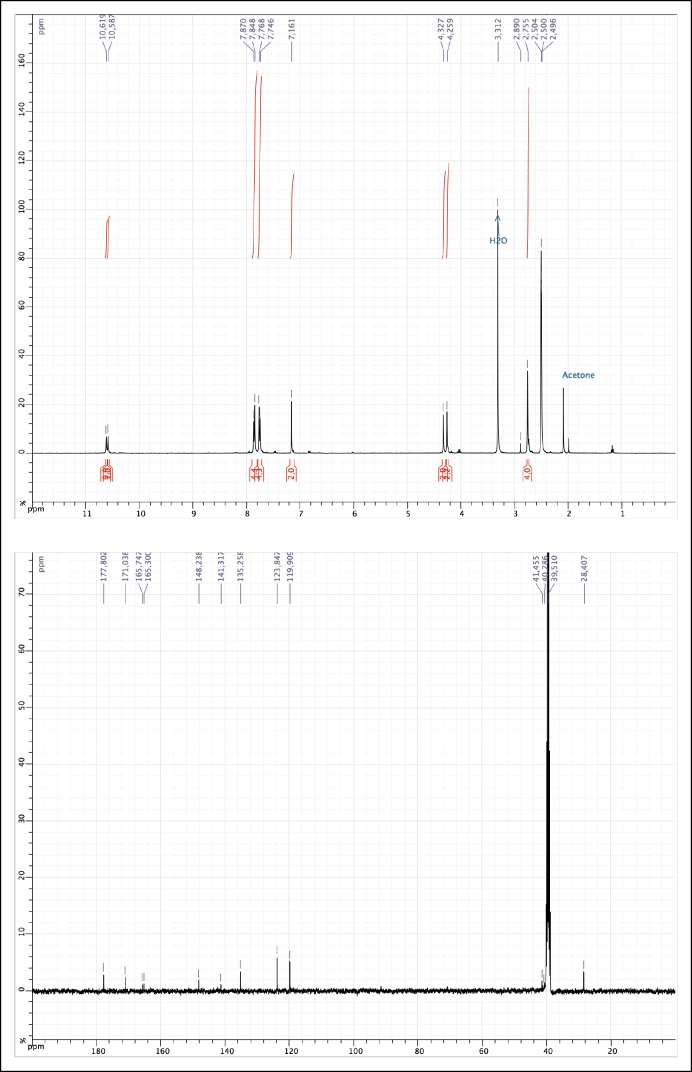
Figure 1—figure supplement 1. Chemical synthesis and physico-chemical properties of azobenzene derivatives.
Figure 1—figure supplement 2. Horizontal screening confirms an outward expansion of the TM helices.