Abstract
The histone demethylase LSD1 (KDM1A) demethylates mono- and di-methylated (Me2) lysine (K) 4 on histone H3. High LSD1 expression blocks differentiation and confers a poor prognosis in AML. Here, treatment with the novel LSD1 antagonist SP2509 attenuated the binding of LSD1 with the co-repressor CoREST, increased the permissive H3K4Me3 mark on the target gene promoters, and increased the levels of p21, p27 and C/EBPα in cultured AML cells. Additionally, SP2509 treatment or LSD1 shRNA inhibited the colony growth of AML cells. SP2509 also induced morphologic features of differentiation in the cultured and primary AML blasts. SP2509 induced more apoptosis of AML cells expressing mutant NPM1 than MLL fusion oncoproteins. Treatment with SP2509 alone significantly improved the survival of immune-depleted mice following tail-vein infusion and engraftment of cultured or primary human AML cells. Co-treatment with pan-HDAC inhibitor (HDI) panobinostat (PS) and SP2509 was synergistically lethal against cultured and primary AML blasts. Compared to each agent alone, co-treatment with SP2509 and PS significantly improved the survival of the mice engrafted with the human AML cells, without exhibiting any toxicity. Collectively, these findings show that the combination of LSD1 antagonist and pan-HDI is a promising therapy warranting further testing against AML.
Keywords: KDM1A, histone deacetylase inhibitor, acute myeloid leukemia, differentiation
Introduction
Following standard chemotherapy, while complete remissions are routinely achieved, a majority of patients with acute myeloid leukemia (AML) eventually suffer relapse with treatment-refractory disease (1). Consequently, the overall five year survival of AML patients remains approximately 23%, creating a compelling rationale to develop novel therapeutics for AML (2). In the pathogenesis of AML, multiple mechanisms involving genetic alterations and epigenetic deregulations collaborate to cause aberrant maturation arrest, growth and survival of early myeloid progenitor cells (3,4). Among the deregulated epigenetic mechanisms, in addition to DNA methylation and histone (H) de-acetylation, alterations in histone H3 lysine (K)-specific methylation are involved in promoting the aberrant gene expression or ‘transcriptome’ in AML cells, which includes the deregulated expression of oncogenes and tumor suppressor genes (5,6). While the levels H3K27 trimethylation (3Me) and H3K9Me3 are among the repressive chromatin marks, H3K4Me3 is a permissive histone modification that promotes gene transcription (3,6). LSD1 (KDM1A) is an FAD-dependent histone demethylase, with homology to amine oxidases, which demethylates di- and mono-methylated K4 on histone H3, reducing the permissive H3K4Me3 (7,8). LSD1 is known to interact with the co-repressor complex Co-REST, containing REST (RE1-silencing transcription factor) and the histone deacetylases (HDAC) 1 & 2, which augments the gene repressor activity of LSD1 (9,10). High LSD1 expression has been shown to confer poor prognosis in cancers (11,12). LSD1 has also been shown to demethylate non-histone products most notably p53 and DNMT1, which improves their stability (13-15). While the null mutation of LSD1 is embryonically lethal (15), LSD1 inhibition has been shown to attenuate growth of pluripotent cancer cells by repressing OCT4 and SOX2 (16). A recent report demonstrated that LSD1 inhibition increased H3K4Me2 levels and induced the expression of myeloid–differentiation associated genes (17). Co-treatment with the LSD1 inhibitor tranylcypromine (TCP), which also inhibits monoamine oxidase (MAO) A and B, and all-trans retinoic acid (ATRA) was shown to diminish the engraftment of primary AML cells in vivo in the NOD/SCID-IL-2receptor-γ deficient (NSG) mice (17). Also, LSD1 inhibition with a TCP analogue phenocopied LSD1 knockdown in primary AML cells expressing MLL fusion oncoprotein (18). Furthermore, LSD1 was shown to sustain the leukemogenic potential of the MLL-AF9 leukemia stem cells (18). Collectively, these reports strongly suggest that targeted knockdown of LSD1 levels and activity induces differentiation and exerts anti-AML activity. However, in each report, the LSD1 inhibitor used exhibited severe in vivo toxicity at the concentration that inhibited LSD1 activity and reduced the AML burden (17,18). SP2509 is a novel, FAD-binding pocket, non-MAOA and MAOB inhibitor of LSD1 (19). In the present studies, we determined the chromatin-modifying as well as the in vitro and in vivo anti-AML activity of SP2509 against cultured and primary human AML cells. Recently, treatment with a pan-HDAC inhibitor was also shown to down regulate LSD1 thru Sp1 inhibition (20). In AML cells, the pan-HDAC inhibitor panobinostat (PS) was also shown to increase H3K4Me3 plus inhibit H3K27Me3 levels, inducing p21 (CDKN1A), p15 (CDKN2B) and p16 (CDKN2A), as well as inhibiting cell cycle progression and promoting differentiation and apoptosis in AML cells (21,22). Therefore, in the present studies, we also determined the in vitro and in vivo anti-AML activity of co-treatment with SP2509 and PS. Our findings demonstrate that the combined treatment exerts synergistic in vitro activity against cultured and primary AML progenitor/stem cells. Additionally, as compared to each agent alone, co-treatment with SP2509 and PS significantly improved the survival of immune-depleted mice engrafted with cultured or primary human AML cells.
Methods and Materials
Reagents and antibodies
LSD1 antagonist, SP2509, and its inactive enantiomer, SP2513, were kindly provided by Salarius Pharmaceuticals. Panobinostat (PS) was provided by Novartis Pharmaceuticals Inc. (East Hanover, NJ). Anti-H3K4Me3, H3K9Me2 and H3K27Me3 antibodies for chIP were obtained from Millipore (Billirica, MA). Anti-LSD1, cleaved PARP, anti-c-MYC and anti-BIM antibodies were obtained from Cell Signaling (Danvers, MA). Anti-p21WAF antibody was obtained from Neomarkers (Fremont, CA). Anti-p27KIP antibody was obtained from BD Biosciences (San Jose, CA). Anti-CoREST antibody was obtained from Abcam (Cambridge, MA) Anti-β-actin antibody and lentiviral short hairpin RNAs targeting LSD1 or non-targeting shRNA (sh-NT) were obtained from Sigma Aldrich (St. Louis, MO).
SP2509 activity assays
The LSD1 screening biochemical assay kit was purchased from Cayman Chemical (Ann Arbor, MI). Test compounds were diluted to 20× the desired test concentration in 100% DMSO and 2.5 μL of the diluted drug sample was added to a black 384-well plate. The LSD1 enzyme stock was diluted 17-fold with assay buffer and 40 μL of the diluted LSD1 enzyme was added to the appropriate wells. Substrate, consisting of horseradish peroxidase, dimethyl K4 peptide corresponding to the first 21 amino acids of the N-terminal tail of histone H3, and 10-acetyl-3,7-dihydroxyphenoxazine was then added to wells. Resorufin was analyzed on an Envision plate reader with an excitation wavelength of 530 nm and an emission wavelength of 595 nm. The activity of SP2509 on the other oxidases was determined by utilizing commercially available kits. For determining the glucose oxidase (GO) activity (which also non-covalently binds FAD in an elongate conformation), the GO kit utilized was procured from Life Technologies (Carlsbad, CA, Cat No A22189). The monoamine oxidase assays were performed using the MAO-glo kit (Promega V1401) with MAO-A from Promega (V1452) and MAO-B from Sigma (M7441-1VL, St Louis, MO).
Cell Culture
OCI-AML3 and MOLM13 cells were obtained from the DSMZ (Braunschweig, Germany) and cultured in the recommended media. MV4-11 cells were obtained from ATCC (Manassas, VA) and cultured in the recommended media. All experiments with cell lines were performed within 6 months after thawing or obtaining from ATCC or DSMZ. Cell line authentication was performed by ATCC or DSMZ. The ATCC and DSMZ utilize short tandem repeat (STR) profiling for characterization and authentication of cell lines.
Primary normal progenitor and AML BPCs
Primary peripheral blood and/or bone marrow aspirate AML samples were obtained and prepared for the studies below, as previously described (22,23). Banked, de-linked and de-identified, normal or AML CD34+ or AML CD34+CD38-LIN- bone marrow progenitor/stem cells were purified, as previously described (21,22).
Assessment of apoptosis by annexin-V staining
Untreated or drug-treated cells were stained with Annexin-V (Pharmingen, San Diego, CA) and TO-PRO-3 iodide and the percentages of apoptotic cells were determined by flow cytometry, as previously described (23,24). The combination index (CI) for each drug combination and the evaluation of the synergistic interactions were calculated by median dose effect analyses (assuming mutual exclusivity) utilizing the commercially available software Calcusyn (Biosoft, Ferguson, MO) (25). CI values of less than 1.0 represent a synergistic interaction of the two drugs in the combination.
Assessment of percentage non-viable cells
Following designated treatments, cells were washed with 1X PBS, stained with propidium iodide and analyzed by flow cytometry, as previously described (23,24).
Assessment of differentiation and CD86 expression
Following designated treatments, cells were washed with 1X PBS, then incubated in 0.2% BSA/PBS with IgG isotype control, anti-CD11b, anti-CD14 or anti-CD86 antibodies in the dark at 4°C for 30 minutes. Cells were washed with 0.2% BSA/PBS and the % of CD11b, CD14 or CD86 positive cells were determined by flow cytometry (23,26). For determination of CD68 expression, cells were fixed with paraformaldehyde, permeabilized with methanol and incubated in 0.2% BSA/PBS with IgG isotype control or anti-CD68 antibody in the dark at 4°C for 30 minutes. Cells were washed with 0.2% BSA/PBS and the % of CD68 positive cells were determined by flow cytometry. For assessment of morphologic differentiation of cultured and primary AML cells, cells were cytospun onto glass slides for 5 minutes. Cells were stained with hematoxylin and eosin and the cells were observed by light microscopy. The % morphologic differentiation was determined by counting 100-200 cells on the slides (23).
Colony growth assay
Cultured AML cells were treated with JQ1 and/or PS for 48 hours. At the end of treatment, cells were washed free of the drugs and 500 cells per condition were plated in methylcellulose and incubated at 37°C. Colony formation was measured 7-10 days after plating (23,24).
Chromatin immunoprecipitation and Real Time Polymerase Chain Reaction
OCI-AML3 and MOLM13 cells were treated with SP2509 for 16 hours. Following drug exposure, cross-linking, cell lysis, sonication and chromatin immunoprecipitation for 3MeK4 Histone H3, 2MeK9 Histone H3 or 3MeK27 Histone H3 was performed according to the manufacturer's protocol (Millipore). For quantitative assessment of p57KIP, KLF4, and p21 promoter DNA in the chromatin immunoprecipitates, a SYBR Green Mastermix from Applied Biosystems was used (Foster City, CA). Relative enrichment of the promoter DNA in the chromatin immunoprecipitates was normalized against the amount of p57KIP, KLF4, and p21 promoter DNA in the input samples (22,27).
RNA isolation and quantitative polymerase chain reaction
Following the designated treatments with SP2509, total RNA was isolated with a High Pure RNA isolation kit (Roche Diagnostics, Indianapolis, IN) and reverse transcribed. Quantitative real-time PCR analysis for the expression of p57KIP, KLF4, and p21 was performed on cDNA using TaqMan probes from Applied Biosystems (Foster City, CA) (22,23). Relative mRNA expression was normalized to the expression of GAPDH.
Short hairpin RNA to LSD1
Lentiviral short hairpin (sh) RNAs targeting LSD1 or non-targeting shRNA (sh-NT) were procured from Sigma Aldrich (St. Louis, MO) and transduced into OCI-AML3 cells, as previously described (23). Forty-eight hours post transduction, the cells were washed with complete media and plated with or without PS for 48 hours for assessing apoptosis, cell proliferation or colony growth.
Cell lysis, protein quantitation and immunoblot analyses
Untreated or drug-treated cells were centrifuged, and the cell pellets were lysed and the protein quantitation and immunoblot analyses were performed, as previously described (22-24). Immunoblot analyses were performed at least twice and representative immunoblots are shown.
In vivo model of acute myeloid leukemia
All in vivo studies were approved by, and conducted in accordance with the guidelines of the IACUC at Houston Methodist Research Institute. Female NOD/SCID mice were exposed to 2.5 Gy of radiation. The following day, 5 million OCI-AML3 cells were injected into the lateral tail vein of the mice and the mice were monitored for 7 days. Following treatments were administered in cohorts of 8 mice for each treatment: vehicle alone, 25 mg/kg SP2509, 5 mg/kg panobinostat and SP2509 plus panobinostat. Treatments were initiated on day 7 for OCI-AML3 cells. SP2509 (formulated in solubilization buffer [20% Cremaphor, 20% DMSO, 60% sterile water) was administered twice per week (Tues and Thurs) intraperitoneally (IP) for 3 weeks, and then discontinued. Panobinostat (formulated in 5% DMSO/ 95% normal saline) was administered by IP injection 3 days per week (M-W-F) for 3 weeks and discontinued. The survival of mice is represented by a Kaplan Meier survival plot (22, 23). The doses of PS utilized in these studies were determined to be safe and effective through previously reported studies (22). A separate in vivo experiment was conducted utilizing NSG mice and primary AML cells (28,29). Following engraftment of the AML cells (presence of greater than 1% CD45+ cells in the peripheral blood), mice were treated with SP2509 and/or PS, as described above, for three weeks (9,29). The survival of mice is represented by a Kaplan Meier plot.
Statistical Analysis
Significant differences between values obtained in a population of AML cells treated with different experimental conditions were determined using a two-tailed, paired t-test or a one way ANOVA analysis within an analysis package of Microsoft Excel 2010 software or using GraphPad Prism (GraphPad Software, Inc., CA). P values of less than 0.05 were assigned significance. Statistical differences in the survival of the mice treated with SP2509, PS or SP2509 + PS were determined by Log-rank (Mantel-Cox) test. P values of less than 0.05 were assigned significance.
Results
SP2509 inhibits LSD1 activity, reduces colony growth and induces apoptosis of cultured AML cells
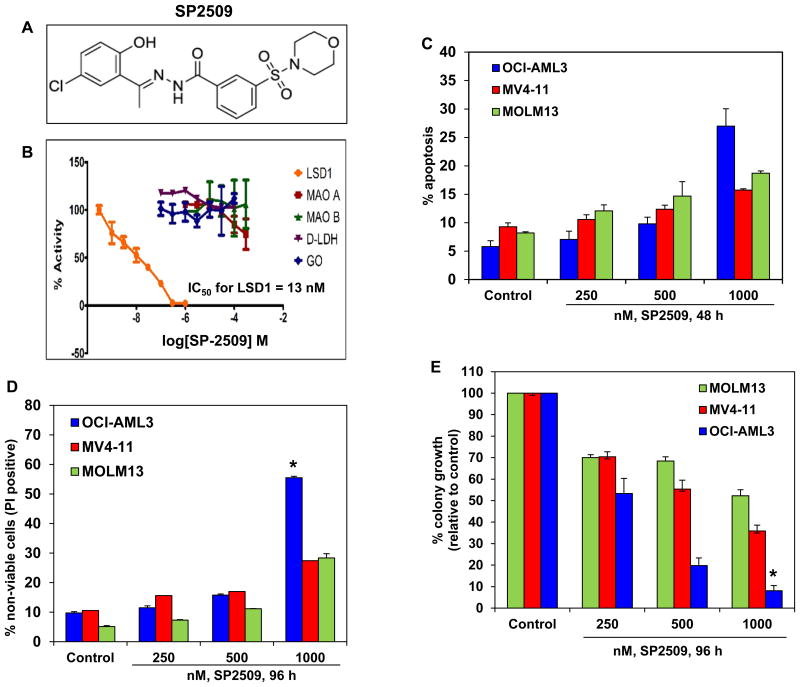
Utilizing a cell-free in vitro assay, we first determined the activity of SP2509 against a variety of oxidases including the monoamine oxidases A and B (MAO A and MAO B) (Figure 1A and 1B). SP2509 was found to selectively inhibit LSD1 at low nanomolar concentrations (Figure 1B). The 50% inhibitory concentration (IC50) of SP2509 against LSD1 was determined to be 13 nM. SP2509 was inactive against MAOA, MAOB, LDH (lactate dehydrogenase and GO (glucose oxidase). We next determined the ability of SP2509 to induce apoptosis in cultured AML cells. As shown in Figure 1C, treatment with SP2509 for 48 hours dose-dependently induced modest levels of apoptosis in AML cells (Figure 1C). However, OCI-AML3 cells, which are known to express mutant NPM1 and DNMT3A (23), were significantly more sensitive than the other AML cell types (p < 0.05). As determined by propidium iodide uptake and flow cytometry, following 96 hours of exposure to SP2509, OCI-AML3 cells also exhibited the highest loss of viability compared to the other AML cell-types (Figure 1D). Treatment with SP2509 also dose-dependently depleted the % of cells positive for Ki67 expression and inhibited the colony growth of OCI-AML3 significantly more than of the other AML cell-types studied (p< 0.01), showing greater than 90% loss of clonogenic survival of OCI-AML3 cells (Figure 1E and Supplemental Figure 1).
Figure 1. Treatment with the LSD1 antagonist, SP2509, inhibits LSD1 activity, depletes colony growth and induces apoptosis and cell death of cultured human acute myeloid leukemia cells.
A. Chemical structure of SP2509. B. In vitro assay demonstrating the low nanomolar activity of SP-2509 for LSD1 compared to other monoamine oxidases (MAO-A and MAO-B), lactate dehydrogenase (D-LDH), and glucose oxidase (GO). C. OCI-AML3, MV4-11, and MOLM13 cells were treated with the indicated concentrations of SP2509 for 48 hours. At the end of treatment, cells were stained with annexin V and TO-PRO-3 iodide and the % of annexin V-positive, apoptotic cells was determined by flow cytometry. Columns, mean of three experiments; Bars, standard error of the mean. D. OCI-AML3, MV4-11, and MOLM13 cells were treated with the indicated concentrations of SP2509 for 96 hours. Then, cells were stained with propidium iodide and the % of non-viable cells was determined by flow cytometry. Columns, mean of three experiments; Bars, standard error of the mean. * indicates values significantly greater in OCI-AML3 cells compared to MOLM13 and MV4-11 cells (p< 0.01). E. OCI-AML3, MV4-11, and MOLM13 cells were treated with the indicated concentrations of SP2509 for 96 hours. At the end of treatment, live cells were plated in methylcellulose and incubated at 37°C for 7-10 days. The % colony growth is reported relative to the untreated cells. Columns, mean of three experiments; Bars, standard error of the mean. * indicates values significantly less in OCI-AML3 cells compared to MOLM13 and MV4-11 cells (p< 0.01).
SP2509 treatment inhibits the association of LSD1 with CoREST, increases promoter-specific H3K4Me3 and induces p53, p21 and CEBPα in AML cells
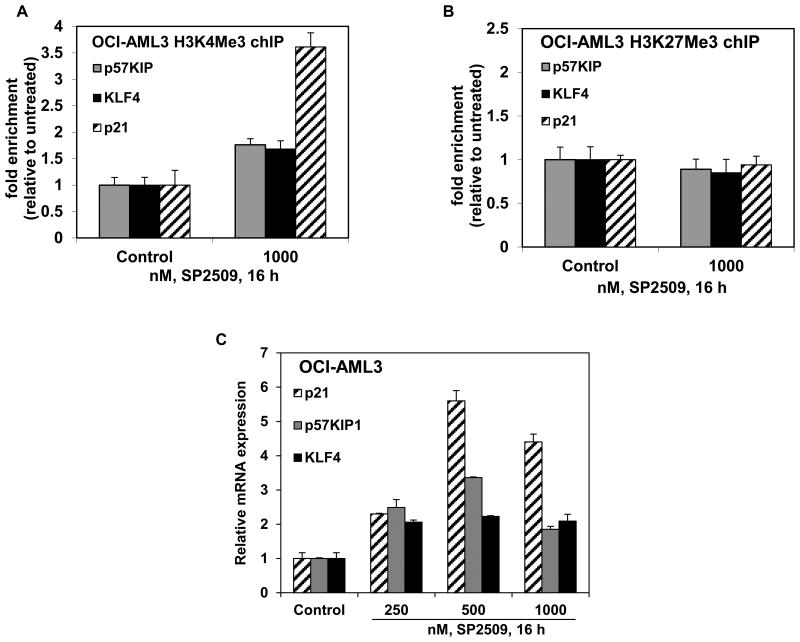
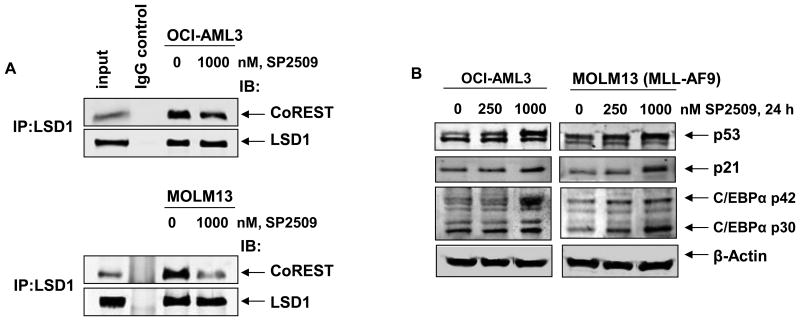
Turning to the chromatin effects of SP2509, utilizing ChIP analysis with anti-H3K4Me3 antibody, we determined the effect of SP2509 treatment on the levels of H3K4Me3 that was associated with the chromatin of the known LSD1 target gene promoters, e.g., those of p57KIP, KLF4 and p21. Treatment with SP2509 increased the level of H3K4Me3 1.7 fold (p57KIP and KLF4) to 3.5 fold (CDKN1A, p21) associated with the chromatin of these gene promoters in OCI-AML3 (Figure 2A). SP2509 treatment also increased the H3K9Me2 levels (Supplemental Figure 2), but did not alter the H3K27Me3 levels associated with the promoters of these genes (Figure 2B). Consistent with this, SP2509 treatment also increased the mRNA levels of p57KIP, KLF4 and p21 in a dose-dependent manner, with the maximum increase observed for the mRNA of p21, following exposure to SP2509 for 16 hours (Figure 2C). Treatment with SP2509 exerted similar effects on the chromatin alterations and the mRNA levels in MOLM13 cells (Supplemental Figure 3A and 3B). We also determined whether treatment with SP2509 affected the association of LSD1 with the co-repressor CoREST in the cultured AML cells. As shown in Figure 3A, SP2509 treatment disrupted the association of LSD1 with CoREST in OCI-AML3 and MOLM13 cells, without affecting the levels of LSD1. Concomitantly, treatment with SP2509 also induced the protein levels of p53 and p21, as well as of the myeloid differentiation-associated transcription factor C/EBPα in the AML cells (Figure 3B).
Figure 2. Treatment with SP2509 increases H3K4Me3 on the promoters of p57 Kip, KLF4, and p21 and induces mRNA expression of p57Kip, KLF4 and p21 in AML cells.
A-B. OCI-AML3 cells were treated with the indicated concentrations of SP2509 for 16 hours. At the end of treatment, chromatin was cross-linked, sonicated, and chromatin immunoprecipitation (chIP) was performed for H3K4Me3 and H3K27Me3. The chIP'ed DNA was used as template for qPCR. The fold enrichment was calculated using the Ct value of the chIP'ed DNA versus the Ct for the input DNA. C. OCI-AML3 cells were treated with the indicated concentrations of SP2509 for 16 hours. Then, total RNA was isolated and reverse transcribed. The resulting cDNA was utilized for quantitative PCR of p57Kip, KLF4 and p21 using TaqMan probes. The relative mRNA expression was normalized against GAPDH.
Figure 3. Treatment with SP2509 inhibits binding of LSD1 with CoREST and induces p53, p21 and C/EBP α in AML cells.
A. OCI-AML3 and MOLM13 cells were treated with the indicated concentration of SP2509 for 16 hours. At the end of treatment, total cell lysates were prepared and LSD1 was immunoprecipitated. After thorough washing, SDS-PAGE and immunoblot analyses were conducted for CoREST and LSD1 in the immunoprecipitates. B. OCI-AML3 and MOLM13 cells were treated with the indicated concentration of SP2509 for 24 hours. Following this, cells were harvested, total cell lysates were prepared and immunoblot analyses were conducted for the expression levels of p53, p21, C/EBPα and β-actin in the lysates.
Treatment with SP2509 induces differentiation of cultured and primary AML cells
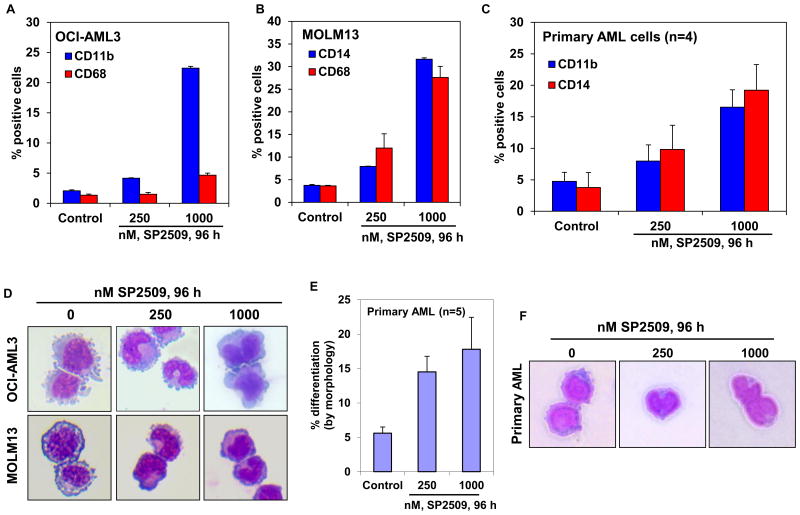
Previous reports have demonstrated that treatment with the non-specific LSD1 antagonist TCP or a biguanide polyamine analog induces myelo-monocytic differentiation-associated markers, as well as induces the morphologic features of differentiation in AML cells (17). Based on this and prompted by the observation that SP2509 treatment induced p21 levels and increased the expression of C/EBPα, we determined the ability of SP2509 to induce differentiation in AML cells. Treatment with SP2509 increased the expression of the myelo-monocytic differentiation marker CD11b, with only a minimal increase in the expression of CD68 in OCI-AML3 cells (Figure 4A). In contrast, exposure to SP2509 increased the expression of CD14 and CD68 in MOLM13 cells (Figure 4B). MOLM13 cell do not express CD11b, and SP2509 treatment did not induce its expression in MOLM13 cells. Unlike what has been previously reported, SP2509 treatment did not increase the expression of CD86 in OCI-AML3 or MOLM13 cells (Supplemental Figure 4) (26). Treatment with SP2509 also increased the expression of CD11b and CD14 in four separate samples of primary AML blasts expressing NPM1c+ and/or FLT3-ITD (Figure 4C). Importantly, SP2509 treatment also induced the morphologic features of differentiation in OCI-AML3 and MOLM13 cells, as exemplified in Figure 4D. Exposure to SP2509 also dose-dependently induced morphologic features of differentiation in the primary AML blasts (Figure 4E), as also exemplified and presented in Figure 4F. Induction of the morphologic features of differentiation is regarded as the ‘gold standard’ of evidence for differentiation in the myeloid lineage AML cells.
Figure 4. Treatment with SP2509 induces features of morphologic differentiation of cultured and primary AML cells.
A-B. OCI-AML3 and MOLM13 cells were treated with the indicated concentrations of SP2509 for 96 hours. At the end of treatment, cells were stained with CD11b, CD14 or CD68 antibodies and the % of cells positive for these markers was determined by flow cytometry. Columns represent the mean of three independent experiments ± S.E.M. C. CD34+ progenitor cells purified from AML blasts (n=4) were treated with the indicated concentrations of SP2509 for 96 hours. Following this, cells were stained with CD11b or CD14 antibodies and the % of cells positive for these markers was determined by flow cytometry. Columns represent the mean % of CD11b or CD14 positive cells ± S.E.M. D. OCI-AML3 and MOLM13 cells were treated with the indicated concentrations of SP2509 for 96 hours. Following this, cells were cytospun onto glass slides and stained with hematoxylin and eosin. Cells were observed at 40× magnification using a light microscope. Images of differentiated cells were obtained using a CCD camera. Representative cells are shown. E. CD34+ progenitor cells purified from AML blasts (n=5) were treated with the indicated concentrations of SP2509 for 96 hours. Then, cells were cytospun onto glass slides and stained with hematoxylin and eosin. The percentages of differentiated cells were determined by light microscopy. Columns, mean morphologic differentiation of the AML samples; Bars, standard error of the mean. F. CD34+ progenitor cells purified from AML blasts were treated with the indicated concentrations of SP2509 for 96 hours. Following this, cells were cytospun onto glass slides and stained with hematoxylin and eosin. Cells were observed at 40× magnification using a light microscope. Images of differentiated AML cells were obtained using a CCD camera.
Targeted knockdown of LSD1 mimics the anti-AML activity of SP2509
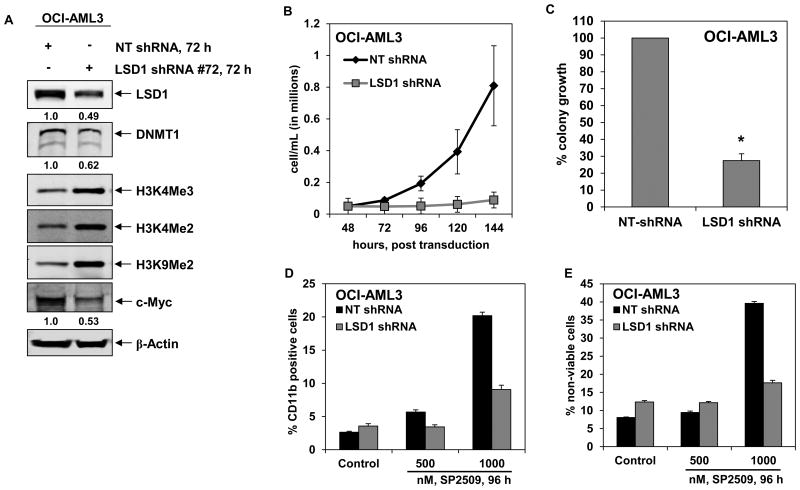
To confirm that the effects of SP2509 on the AML cells was due to SP2509-mediated inhibition of LSD1, we also determined the effect of the shRNA-mediated genetic knock down of LSD1 in OCI-AML3 cells. As compared to the non-targeted (NT) control shRNA, the LSD1 targeted shRNA knocked down the expression of LSD1 by approximately 50% (Figure 5A). Consistent with the previous reports documenting that LSD1 stabilizes DNMT1 levels (15), targeted knock down of LSD1 also depleted DNMT1 in OCI-AML3 cells (Figure 5A). Attenuation of LSD1 was associated with increase in the levels of global histone H3K4Me2, H3K4Me3 and H3K9Me2, but depletion in the levels of c-Myc protein (Figure 5A). Lentiviral transduction of LSD1 shRNA in OCI-AML3 cells resulted in decline in the suspension culture growth over 96 hours (Figure 5B) and colony culture growth in semi-solid medium over 10 days (Figure 5C). It is noteworthy that, compared to OCI-AML3 cells transduced by the NT shRNA, SP2509 treatment-induced expression of CD11b and the loss of viability was significantly abrogated in OCI-AML3 cells transduced by the shRNA to LSD1 (Figure 5D and 5E). This indicated that the differentiation and loss of viability mediated by SP2509 was at least partly mediated through inhibition of LSD1.
Figure 5. Short hairpin RNA-mediated knockdown of LSD1 inhibits cell proliferation, colony growth and significantly enhances pan-HDAC inhibitor panobinostat-induced apoptosis of AML cells.
A. OCI-AML3 cells were transduced with non-targeting (NT) shRNA or LSD1-specific shRNA for 72 hours. Following this, cells were harvested and total cell lysates were prepared. Immunoblot analyses were conducted for the expression levels of LSD1, DNMT1, c-MYC, H3K4Me3, H3K4Me2, H3K9Me2 and β-actin in the cell lysates. B. OCI-AML3 cells were transduced with NT and LSD1 shRNA for 48 hours. Then, cells were counted and 50,000 cells were plated in duplicate. Cell counts were taken every 24 hours for 144 hours post transduction. Values represent the mean of three experiments ± S.E.M. C. OCI-AML3 cells were transduced with NT and LSD1 shRNA for 48 hours. Following this, cells were plated in methylcellulose and incubated for 7-10 days. Total number of colonies was counted and the % colony growth is calculated relative to the NT shRNA-transduced cells. * indicates colony growth values significantly less in LSD1 shRNA-transduced cells compared to NT shRNA-transduced cells (p< 0.001). D-E. OCI-AML3 cells were transduced with NT and LSD1 shRNA for 48 hours. Then, cells were treated with the indicated concentrations of SP2509 for 96 hours and the % of CD11b positive cells (D) and non-viable cells (E) were determined by flow cytometry. Columns, mean of three experiments; Bars, standard error of the mean.
Co-treatment with SP2509 and PS exerts synergistic lethal activity against cultured and primary AML cells
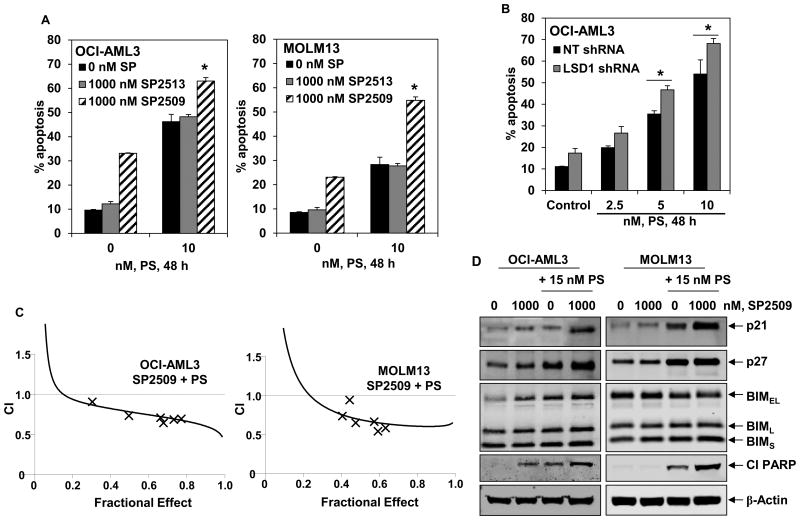
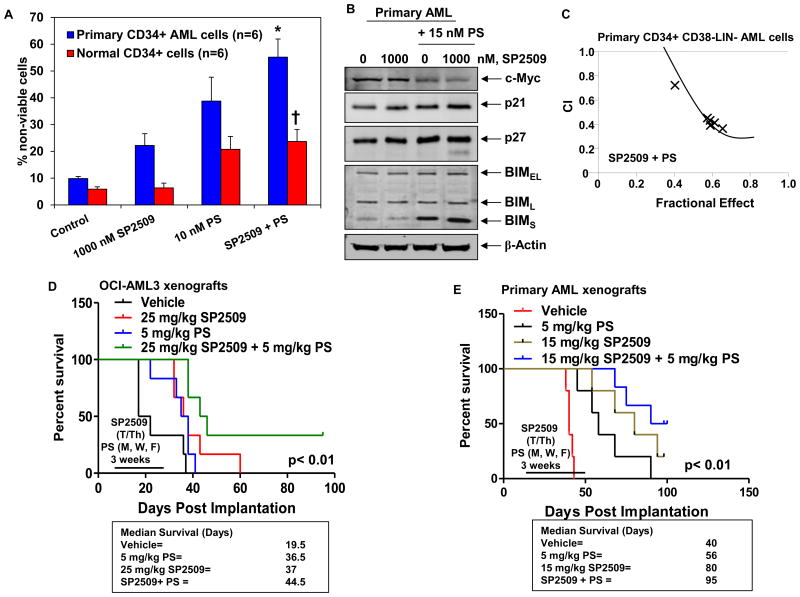
Next, based on the observation that pan-HDIs induce differentiation and apoptosis, as well as deplete LSD1 levels in AML cells (20,22), we determined the effects of co-treatment with PS on the anti-AML activity of SP2509. In these studies, we utilized relatively lower concentrations of PS that are well-tolerated (5 to 15 nM), but biologically effective as epigenetic modifiers (30,31). Figure 6A demonstrates that co-treatment with 10 nM of PS significantly enhanced SP2509-induced but not SP2513 (the inactive enantiomer of SP2509)-induced apoptosis, in OCI-AML3 and MOLM13 cells. Consistent with this observation, treatment with low concentrations of PS induced more apoptosis in OCI-AML3 cells transduced with shRNA to LSD1, as compared to the OCI-AML3 cells transduced with the NT shRNA (Figure 6B). Importantly, combined therapy with PS and SP2509 synergistically induced apoptosis of OCI-AML3, MOLM13 and MV4-11 cells, with combination indices below 1.0 as determined by isobologram analysis (Figure 6C and Supplemental Figure 5A-5C). As compared to treatment with each agent alone, co-treatment with PS and SP2509 was also associated with greater increase in the levels of p21, p27, BIM and cleaved PARP (Figure 6D). We also determined whether exposure to PS and SP2509 more than each agent alone induced biomarkers associated with induction of myeloid differentiation in the cultured AML cells. As shown in Supplemental Figure 6 A and 6B, compared to treatment with each agent alone, co-treatment with SP2509 and PS for 96 hours induced higher levels of p21, p16 and CEBPα, as well as induced higher levels of CD11b in OCI-AML3 cells (p < 0.05). We next determined the lethal effects of SP2509 and/or PS against patient-derived, CD34+ primary AML versus normal bone marrow progenitor cells. Figure 7A demonstrates that treatment with either SP2509 or PS induced more loss of cell viability of the primary AML versus normal progenitor cells. Furthermore, co-treatment with the combination was significantly more lethal against AML versus normal progenitor cells (p < 0.01) (Figure 7A). Additionally, compared to each agent alone, co-treatment with SP2509 and PS caused more loss of viability of the primary AML progenitor cells. This was associated with greater depletion of c-Myc but induction of p21, p27 and BIM in the AML progenitor cells (Figure 7B). We next determined whether the combination was also highly active against the purified CD34+CD38-LIN- primary AML stem/progenitor cells. As shown in Figure 7C and Supplemental Figure 5D, co-treatment with SP2509 and PS was synergistically lethal against the AML stem/progenitor cells.
Figure 6. Combined treatment with SP2509, but not its inactive enantiomer SP2513, and PS synergistically induces apoptosis of cultured AML cells.
A. OCI-AML3 and MOLM13 were treated with the indicated concentrations of SP2509, SP2513 and/or PS for 48 hours. At the end of treatment, cells were stained with annexin V and TO-PRO-3 iodide. The % of annexin V-positive, apoptotic cells was determined by flow cytometry. Columns, mean of three experiments; Bars, standard error of the mean. * indicates values significantly greater in SP2509 and PS treated cells compared to those treated with SP2513 and PS (p < 0.05). B. OCI-AML3 cells were transduced with NT and LSD1 shRNA for 48 hours. Then, cells were treated for an additional 48 hours with the indicated concentrations of PS and the % annexin V positive, apoptotic cells were determined by flow cytometry. Columns, mean of three experiments; Bars, standard error of the mean. * indicates values significantly greater in LSD1 shRNA transduced cells treated with PS (p < 0.05). C. OCI-AML3 and MOLM13 cells were treated with SP2509 (dose range 500-1000 nM) and/or PS (dose range 5-20 nM) at a constant ratio for 48 hours. Following this, cells were stained with annexin V and TO-PRO-3 iodide and the % annexin V-positive, apoptotic cells was determined by flow cytometry. Median dose effect and isobologram analyses were performed using Calcusyn. Combination index (CI) values less than 1.0 indicate a synergistic interaction of the two agents in the combination. D. OCI-AML3 and MOLM13 cells were treated with the indicated concentrations of SP2509 and/or PS for 24 hours. At the end of treatment, cell lysates were prepared and immunoblot analyses were performed as indicated. The expression levels of β-actin in the lysates served as the loading control.
Figure 7. Treatment with SP2509 and/or PS significantly enhances PS-mediated loss of viability of CD34+ primary AML cells and improves the survival of mice bearing AML xenografts and primagrafts.
A. CD34+ progenitor cells purified from AML blasts and normal CD34+ cells were treated with the indicated concentrations of SP2509 and/or PS for 48 hours. At the end of treatment, cells were stained with propidium iodide. The % of non-viable cells was determined by flow cytometry. * indicates values significantly greater in CD34+ AML cells treated with the combination of SP2509 and PS, compared to treatment with either agent alone (p < 0.01). † indicates values significantly less in normal CD34+ cells treated with the combination of SP2509 and PS compared to CD34+ AML cells (p < 0.05). B. Primary AML cells were treated with SP2509 and/or PS as indicated for 24 hours. Total cell lysates were prepared and immunoblot analyses were conducted for the expression levels of c-Myc, p21, p27, BIM, and β-actin in the lysates. C. CD34+CD38-Lin- progenitor cells purified from AML blasts were treated with SP2509 and/or PS for 48 hours. Then, cells were stained with propidium iodide and the % of non-viable cells was determined by flow cytometry. Median dose effect and isobologram analyses were conducted utilizing Calcusyn. CI values less than 1.0 indicate a synergistic interaction of the two agents in the combination. D. NOD/SCID mice which had been exposed to 2.5 Gy of gamma irradiation were injected with 5 million OCI-AML3 cells via the lateral tail vein. Seven days after implantation, mice were treated with 25 mg/kg of SP2509 and/or 5 mg/kg of PS for three weeks. Survival of the mice is represented by a Kaplan Meier plot. E. NSG mice which had been preconditioned with 2.5 Gy of gamma irradiation were injected with 10 million primary AML cells. Treatment was initiated when mice demonstrated 1% CD45+ cells in the peripheral blood. Mice were treated with SP2509 and/or PS, as indicated, for three weeks. Survival of the mice is represented by a Kaplan Meier plot.
Combined treatment with SP2509 and PS exerts superior in vivo activity against AML xenografts and ‘primagrafts’
We next determined the in vivo anti-AML activity of SP2509 and/or PS against cultured (OCI-AML3) in NOD/SCID mice, as well as against primary AML blasts engrafted in the bone marrow, i.e., ‘primagrafts’ in the NSG mice (29). Following the tail vein infusion and engraftment of OCI-AML3 cells, the effect of the treatment with the vehicle control, PS or SP2509 alone, or the combination of SP2509 and PS, for 3 weeks on the survival of the NOD/SCID mice was determined. The Kaplan Meier plot depicting the survival of the mice demonstrates that, as compared to treatment with the vehicle alone, treatment with either SP2509 or PS significantly improved the median survival of the mice infused with OCI-AML3 cells (37 and 36.5 days, respectively, versus 19.5 days for vehicle control) (p < 0.05) (Figure 7D). Notably, combined treatment with PS and SP2509 further improved the median survival of the mice (44.5 days), as compared to treatment with SP2509 or PS alone (p < 0.01). One-third of the mice co-treated with SP2509 and PS were alive 80 days following the infusion of the AML cells. Figure 7E demonstrates the effects of SP2509 and/or PS on the survival of NSG mice engrafted with the primary AML blasts co-expressing FLT3-ITD and NPM1c+. As shown, treatment with SP2509 or PS alone for 3 weeks significantly improved the median survival of the mice, as compared to the treatment with vehicle control (p < 0.01). Combined therapy with SP2509 and PS was superior to the treatment with SP2509 or PS alone in improving the median survival of the mice (95 versus 80 and 56 days, respectively) (p < 0.01), with 50% of the mice surviving more than 100 days after the infusion of the AML blasts in the tail veins of the mice. This plateau in the survival curve suggests a potentially curative impact of the combination on the survival of the mice (Figure 7E). We also determined the ex vivo sensitivity of the primary AML sample utilized as the primagraft in the NSG mice. As shown in Supplemental Figure 7A, co-treatment with SP2509 and PS induced more apoptosis than either agent alone. Ex vivo co-treatment with SP2509 and PS also synergistically induced apoptosis of the primary AML cells with combination indices < 1.0, as determined by median dose effect and isobologram analysis (Supplemental Figure 7B).
Discussion
In the present studies, we demonstrate for the first time that a novel, potent LSD1 antagonist SP2509, induces growth inhibition, differentiation and apoptosis in human AML cells. Importantly, unlike the reported experience with the tranylcypromine analogues, exposure to SP2509 did not induce toxicity in immune depleted mice engrafted with AML at doses that exerted anti-AML activity. Consistent with the previous report demonstrating that LSD1 is essential for the survival of MLL-AF9 expressing leukemia stem cells, our findings show that inhibition of LSD1 is lethal against AML expressing MLL fusion oncoprotein. Moreover, our studies also demonstrate that SP2509 is relatively more active in inducing loss of viability of OCI-AML3 cells expressing mutant NPM1, which had not been previously reported. SP2509 treatment also exhibited in vivo anti-AML activity in immune-depleted mice engrafted with OCI-AML3 cells or primary AML blasts expressing mutant NPM1 plus FLT3-ITD, without inducing any toxicity in the mice, which was noted with the use of tranylcypromine or its analogues as the LSD1 antagonist (17,18).
SP2509 is a small molecule reversible inhibitor of LSD1 (19). It showed activity at low nano-molar levels against LSD1 in the cell-free in vitro enzyme assay, but exerted the biologic activity in the AML cells at the micro-molar level. This is similar to what has been previously reported (19). Unlike the situation in the cell-free enzyme assay, the lower intracellular potency of SP2509 is likely due to the presence and activity of LSD1 in the regulatory protein complexes in the intracellular setting, and the potential regulation of the biologic outcome of LSD1 inhibition by other intracellular factors (9,10,19). For example, the demethylase activity of LSD1 at the nucleosomes is enhanced by its association with the co-repressor complexes such as Co-REST and NURD (9,19,32). Our studies also show that the binding of LSD1 and CoREST is partially disrupted by SP2509 treatment. Inhibition of LSD1 by SP2509 resulted in an increase in the promoter-specific, permissive H3K4Me3 modification, as well as induced p53, p21 and C/EBPα levels. Whether this was due to transcriptional and/or post-transcriptional effects was not determined here; however, these alterations due to SP2509 treatment were clearly associated with growth inhibition, differentiation and the loss of viability of the AML cells. An inverse correlation of LSD1 levels with differentiation has also been reported in neuroblastoma cells (33). In a separate report, alteration in the expression of the Myc ‘core module’ genes was identified in the leukemia stem cells in which LSD1 was knocked down (18). In this report, the knockdown and inhibition of LSD1 was also shown to inhibit growth and induce differentiation of AML progenitor cells expressing MLL fusion oncoprotein (18). We also determined that the biologic effects of SP2509 treatment were also phenocopied by shRNA mediated knock-down of LSD1 in AML cells, suggesting that the effects of SP2509 were mediated by LSD1 inhibition. Recent reports have highlighted that the NPM1 mutation is the founder mutation in the AML stem/progenitor cells where acquisition of FLT3-ITD mutation results in the emergence of an aggressive AML phenotype (4,34). In our studies, SP2509 exerted a relatively higher level of activity against AML cells expressing NPM1 mutation. While the precise mechanism underlying this was not further probed here, a clear role of LSD1-dependent demethylase activity has been demonstrated in normal differentiation and in regulating cell death upon differentiation of stem/progenitor cells (14,35,36). Additionally, LSD1-associated co-repressor complexes have been implicated in TAL1 and Notch signaling, which are also involved in regulating the biology of hematopoietic stem/progenitor cells (37-39). Consistent with this, our findings show that SP2509 exerted in vitro and in vivo anti-AML activity especially against the leukemia-initiating primary AML progenitor/stem cells co-expressing NPM1 and FLT3 mutations.
A previous study demonstrated that co-treatment with LSD1 antagonist enhanced the differentiation inducing activity of all-trans retinoic acid (ATRA) against AML cells (17). LSD1 associated CoREST and NURD complexes also contain class I HDAC-associated HDAC activity (9,10,19,32). Consistent with this, and with our previously reported findings that treatment with pan-HDI PS induces differentiation in AML blast progenitor cells (22), our present in vitro findings confirm that co-treatment with SP2509 or knockdown of LSD1 by shRNA enhanced the lethal effects of relatively low and clinically achievable concentrations of PS in AML cells (30,31). We also demonstrate here that co-treatment with SP2509 and PS was associated with greater induction of the markers of myeloid differentiation in AML cell, which could potentially contribute to the superior anti-AML activity of the combination. Moreover, we observed synergistic apoptotic effects of SP2509 and PS against cultured and primary AML cells. This may be explained by greater induction of the levels of pro-apoptotic proteins p27 and BIM in AML cells exposed to co-treatment with SP2509 and PS, as compared to each drug alone (40,41). Previous reports have identified that LSD1-mediated histone demethylation triggers Myc-induced transcription, and the Myc ‘core module’ gene expression was altered in the leukemia stem cells in which LSD1 was knocked down (18,42). Here, we also noted that synergistic activity of co-treatment with SP2509 and PS was associated with a greater depletion of c-Myc expression in the primary AML blast progenitor cells expressing mutant NPM1. While other reports have shown that combined inhibition of LSD1 and HDACs achieves superior anti-tumor activity against glioblastoma and breast cancer cells (43,44), in these reports, the LSD1 antagonists employed are known to be either irreversible LSD1 inhibitors (45), or exhibit intolerable in vivo activity in the mouse models of AML where these inhibitors were studied (17,18). In contrast, our in vivo studies demonstrate that co-treatment with SP2509 and PS yields significantly better survival without inducing toxicity, and suggests a potential for cure of the immune-depleted mice engrafted with AML cells expressing NPM1 mutation irrespective of the co-expression of FLT3-ITD. Collectively, these findings strongly support the rationale for further in vivo testing of SP2509-based combination with pan-HDAC inhibitor such as PS against AML, especially those expressing NPM1 mutation.
Supplementary Material
Footnotes
Authorship: W. F., B. S., B.P.P, S. G.T. D., and K. L. performed in vitro experiments in cultured and primary AML cells and analyzed the data. W. F. performed the in vivo studies in the NOD/SCID and NSG mice. S. S. and D. J. B. provided a critical new reagent for the studies. S. I. provided primary AML cells for the in vitro and in vivo studies. K.N.B. conceptualized, planned the experiments, supervised the studies, analyzed the data and prepared the manuscript.
Conflict of interest: David Bearss is founder and Chief Scientific Officer of Salarius Pharmaceuticals; Sunil Sharma is Chief Medical Officer of Salarius Pharmaceuticals. All other authors have no relevant financial interests to disclose.
Supplementary information is available at Leukemia's website.
References
- 1.Ravandi F. Novel treatments in acute myeloid leukemia. Clin Adv Hematol Oncol. 2011;9(4):333–334. [PubMed] [Google Scholar]
- 2.American Cancer Society. Atlanta: American Cancer Society; What are the key statistics about acute myeloid leukemia? Internet. [updated 2013 Sept. 23; cited 2013 Nov 29]. Available from: http://www.cancer.org/Cancer/Leukemia-AcuteMyeloidAML/DetailedGuide/leukemia-acute-myeloid-myelogenous-key-statistics. [Google Scholar]
- 3.Shen H, Laird PW. Interplay between the cancer genome and epigenome. Cell. 2013;153(1):38–55. doi: 10.1016/j.cell.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welch JS, Ley TJ, Link DC, Miller CA, Larson DE, Koboldt DC, et al. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012;150(2):264–278. doi: 10.1016/j.cell.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdel-Wahab O, Levine RL. Mutations in epigenetic modifiers in the pathogenesis and therapy of acute myeloid leukemia. Blood. 2013;121(18):3563–3572. doi: 10.1182/blood-2013-01-451781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arrowsmith CH, Bountra C, Fish PV, Lee K, Schapira M. Epigenetic protein families: a new frontier for drug discovery. Nat Rev Drug Discov. 2012;11(5):384–400. doi: 10.1038/nrd3674. [DOI] [PubMed] [Google Scholar]
- 7.Hou H, Yu H. Structural insights into histone lysine demethylation. Curr Opin Struct Biol. 2010;20(6):739–748. doi: 10.1016/j.sbi.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kooistra SM, Helin K. Molecular mechanisms and potential functions of histone demethylases. Nat Rev Mol Cell Biol. 2012;13(5):297–311. doi: 10.1038/nrm3327. [DOI] [PubMed] [Google Scholar]
- 9.Shi YJ, Matson C, Lan F, Iwase S, Baba T, Shi Y. Regulation of LSD1 histone demethylase activity by its associated factors. Mol Cell. 2005;19(6):857–864. doi: 10.1016/j.molcel.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 10.Forneris F, Binda C, Dall'Aglio A, Fraaije MW, Battaglioli E, Mattevi A. A highly specific mechanism of histone H3-K4 recognition by histone demethylase LSD1. J Biol Chem. 2006;281(46):35289–35295. doi: 10.1074/jbc.M607411200. [DOI] [PubMed] [Google Scholar]
- 11.Lv T, Yuan D, Miao X, Lv Y, Zhan P, Shen X, Song Y. Over-expression of LSD1 promotes proliferation, migration and invasion in non-small cell lung cancer. PLoS One. 2012;7(4):e35065. doi: 10.1371/journal.pone.0035065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao ZK, Yu HF, Wang DR, Dong P, Chen L, Wu WG, et al. Overexpression of lysine specific demethylase 1 predicts worse prognosis in primary hepatocellular carcinoma patients. World J Gastroenterol. 2012;18(45):6651–6656. doi: 10.3748/wjg.v18.i45.6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang J, Sengupta R, Espejo AB, Lee MG, Dorsey JA, Richter M, et al. p53 is regulated by the lysine demethylase LSD1. Nature. 2007;449(7158):105–108. doi: 10.1038/nature06092. [DOI] [PubMed] [Google Scholar]
- 14.Amente S, Lania L, Majello B. The histone LSD1 demethylase in stemness and cancer transcription programs. Biochim Biophys Acta. 2013;1829(10):981–986. doi: 10.1016/j.bbagrm.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Hevi S, Kurash JK, Lei H, Gay F, Bajko J, et al. The lysine demethylase LSD1 (KDM1) is required for maintenance of global DNA methylation. Nat Genet. 2009;41(1):125–129. doi: 10.1038/ng.268. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Lu F, Ren Q, Sun H, Xu Z, Lan R, et al. Novel histone demethylase LSD1 inhibitors selectively target cancer cells with pluripotent stem cell properties. Cancer Res. 2011;71(23):7238–7249. doi: 10.1158/0008-5472.CAN-11-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schenk T, Chen WC, Gollner S, Howell L, Jin L, Hebestreit K, et al. Inhibition of the LSD1 (KDM1A) demethylase reactivates the all-trans-retinoic acid differentiation pathway in acute myeloid leukemia. Nat Med. 2012;18(4):605–611. doi: 10.1038/nm.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris WJ, Huang X, Lynch JT, Spencer GJ, Hitchin JR, Li Y, et al. The histone demethylase KDM1A sustains the oncogenic potential of MLL-AF9 leukemia stem cells. Cancer Cell. 2012;21(4):473–487. doi: 10.1016/j.ccr.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Sankar S, Bell R, Stephens B, Zhuo R, Sharma S, Bearss DJ, et al. Mechanism and relevance of EWS/FLI-mediated transcriptional repression in Ewing sarcoma. Oncogene. 2013;32(42):5089–5100. doi: 10.1038/onc.2012.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang PH, Chen CH, Chou CC, Sargeant AM, Kulp SK, Teng CM, et al. Histone deacetylase inhibitors stimulate histone H3 lysine 4 methylation in part via transcriptional repression of histone H3 lysine 4 demethylases. Mol Pharmacol. 2011;79(1):197–206. doi: 10.1124/mol.110.067702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.George P, Bali P, Annavarapu S, Scuto A, Fiskus W, Guo F, et al. Combination of the histone deacetylase inhibitor LBH589 and the hsp90 inhibitor 17-AAG is highly active against human CML-BC cells and AML cells with activating mutation of FLT-3. Blood. 2005;105(4):1768–1776. doi: 10.1182/blood-2004-09-3413. [DOI] [PubMed] [Google Scholar]
- 22.Fiskus W, Wang Y, Sreekumar A, Buckley KM, Shi H, Jillella A, et al. Combined epigenetic therapy with the histone methyltransferase EZH2 inhibitor 3-deazaneplanocin A and the histone deacetylase inhibitor panobinostat against human AML cells. Blood. 2009;114(13):2733–2743. doi: 10.1182/blood-2009-03-213496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balusu R, Fiskus W, Rao R, Chong DG, Nalluri S, Mudunuru U, et al. Targeting levels or oligomerization of nucleophosmin 1 induces differentiation and loss of survival of human AML cells with mutant NPM1. Blood. 2011;118(11):3096–3106. doi: 10.1182/blood-2010-09-309674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiskus W, Verstovsek S, Manshouri T, Smith JE, Peth K, Abhyankar S, et al. Dual PI3K/AKT/mTOR inhibitor BEZ235 synergistically enhances the activity of JAK2 inhibitor against cultured and primary human myeloproliferative neoplasm cells. Mol Cancer Ther. 2013;12(5):577–588. doi: 10.1158/1535-7163.MCT-12-0862. [DOI] [PubMed] [Google Scholar]
- 25.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 26.Lynch JT, Cockerill MJ, Hitchin JR, Wiseman DH, Somervaille TC. CD86 expression as a surrogate cellular biomarker for pharmacological inhibition of the histone demethylase lysine-specific demethylase 1. Anal Biochem. 2013;442(1):104–6. doi: 10.1016/j.ab.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 27.Fiskus W, Rao R, Balusu R, Ganguly S, Tao J, Sotomayor E, et al. Superior efficacy of a combined epigenetic therapy against human mantle cell lymphoma cells. Clin Cancer Res. 2012;18(22):6227–6238. doi: 10.1158/1078-0432.CCR-12-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agliano A, Martin-Padura I, Mancuso P, Marighetti P, Rabascio C, Pruneri G, et al. Human acute leukemia cells injected in NOD/LtSz-scid/IL-2Rgamma null mice generate a faster and more efficient disease compared to other NOD/scid-related strains. Int J Cancer. 2008;123(9):2222–2227. doi: 10.1002/ijc.23772. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez PV, Perry RL, Sarry JE, Perl AE, Murphy K, Swider CR, et al. A robust xenotransplantation model for acute myeloid leukemia. Leukemia. 2009;23(11):2109–2117. doi: 10.1038/leu.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deangelo DJ, Spencer A, Bhalla KN, Prince HM, Fischer T, Kindler T, et al. Phase Ia/II, two-arm, open-label, dose-escalation study of oral panobinostat administered via two dosing schedules in patients with advanced hematologic malignancies. Leukemia. 2013;27(8):1628–1636. doi: 10.1038/leu.2013.38. [DOI] [PubMed] [Google Scholar]
- 31.DeAngelo DJ, Mesa RA, Fiskus W, Tefferi A, Paley C, Wadleigh M, et al. Phase II trial of panobinostat, an oral pan-deacetylase inhibitor in patients with primary myelofibrosis, post-essential thrombocythaemia, and post-polycythaemia vera myelofibrosis. Br J Haematol. 2013;162(3):326–335. doi: 10.1111/bjh.12384. [DOI] [PubMed] [Google Scholar]
- 32.Yang M, Gocke CB, Luo X, Borek D, Tomchick DR, Machius M, et al. Structural basis for CoREST-dependent demethylation of nucleosomes by the human LSD1 histone demethylase. Mol Cell. 2006;23(3):377–387. doi: 10.1016/j.molcel.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Schulte JH, Lim S, Schramm A, Friedrichs N, Koster J, Versteeg R, et al. Lysine-specific demethylase 1 is strongly expressed in poorly differentiated neuroblastoma: implications for therapy. Cancer Res. 2009;69(5):2065–2071. doi: 10.1158/0008-5472.CAN-08-1735. [DOI] [PubMed] [Google Scholar]
- 34.Vassiliou GS, Cooper JL, Rad R, Li J, Rice S, Uren A, et al. Mutant nucleophosmin and cooperating pathways drive leukemia initiation and progression in mice. Nat Genet. 2011;43(5):470–475. doi: 10.1038/ng.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adamo A, Sese B, Boue S, Castano J, Paramonov I, Barrero MJ, et al. LSD1 regulates the balance between self-renewal and differentiation in human embryonic stem cells. Nat Cell Biol. 2011;13(6):652–659. doi: 10.1038/ncb2246. [DOI] [PubMed] [Google Scholar]
- 36.Whyte WA, Bilodeau S, Orlando DA, Hoke HA, Frampton GM, Foster CT, et al. Enhancer decommissioning by LSD1 during embryonic stem cell differentiation. Nature. 2012;482(7384):221–225. doi: 10.1038/nature10805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu X, Li X, Valverde K, Fu X, Noguchi C, Qiu Y, et al. LSD1-mediated epigenetic modification is required for TAL1 function and hematopoiesis. Proc Natl Acad Sci U S A. 2009;106(25):10141–10146. doi: 10.1073/pnas.0900437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Deng C, Hu X, Patel B, Fu X, Qiu Y, et al. Dynamic interaction between TAL1 oncoprotein and LSD1 regulates TAL1 function in hematopoiesis and leukemogenesis. Oncogene. 2012;31(48):5007–5018. doi: 10.1038/onc.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mulligan P, Yang F, Di Stefano L, Ji JY, Ouyang J, Nishikawa JL, et al. A SIRT1-LSD1 corepressor complex regulates Notch target gene expression and development. Mol Cell. 2011;42(5):689–699. doi: 10.1016/j.molcel.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borriello A, Bencivenga D, Criscuolo M, Caldarelli I, Cucciolla V, Tramontano A, et al. Targeting p27Kip1 protein: its relevance in the therapy of human cancer. Expert Opin Ther Targets. 2011;15(6):677–693. doi: 10.1517/14728222.2011.561318. [DOI] [PubMed] [Google Scholar]
- 41.Matthews GM, Newbold A, Johnstone RW. Intrinsic and extrinsic apoptotic pathway signaling as determinants of histone deacetylase inhibitor antitumor activity. Adv Cancer Res. 2012;116:165–197. doi: 10.1016/B978-0-12-394387-3.00005-7. [DOI] [PubMed] [Google Scholar]
- 42.Singh MM, Manton CA, Bhat KP, Tsai WW, Aldape K, Barton MC, et al. Inhibition of LSD1 sensitizes glioblastoma cells to histone deacetylase inhibitors. Neuro Oncol. 2011;13(8):894–903. doi: 10.1093/neuonc/nor049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang Y, Vasilatos SN, Boric L, Shaw PG, Davidson NE. Inhibitors of histone demethylation and histone deacetylation cooperate in regulating gene expression and inhibiting growth in human breast cancer cells. Breast Cancer Res Treat. 2012;131(3):777–789. doi: 10.1007/s10549-011-1480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Culhane JC, Cole PA. LSD1 and the chemistry of histone demethylation. Curr Opin Chem Biol. 2007;11(5):561–568. doi: 10.1016/j.cbpa.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amente S, Bertoni A, Morano A, Lania L, Avvedimento EV, Majello B. LSD1-mediated demethylation of histone H3 lysine 4 triggers Myc-induced transcription. Oncogene. 2010;29(25):3691–3702. doi: 10.1038/onc.2010.120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.