Abstract
Background and Purpose
Neurological deterioration (ND) is a devastating complication following intracerebral hemorrhage (ICH) but little is known about time course and predictors.
Methods
We performed a retrospective cohort study of placebo patients in ICH trials. We performed CT scans within 3 hours of symptoms and at 24- and 72-hours; and clinical evaluations at baseline, 1-hour, and days 1, 2, 3, and 15. Timing of ND was predefined: hyperacute (within 1 hour), acute (1-24 hours), subacute (1-3 days), and delayed (3-15 days).
Results
We enrolled 376 patients and 176 (47%) had ND within 15 days. In multivariate analyses of ND by category, hyperacute ND was associated with hematoma expansion (OR 3.6, 95% CI 1.7-7.6) and baseline ICH volume (OR 1.04 per mL, 95% CI 1.02-1.06) ; acute ND with hematoma expansion (OR 7.59, 95% CI 3.91-14.74), baseline ICH volume (OR 1.02 per mL, 95% CI 1.01-1.04), admission GCS (OR 0.77 per point, 95% CI 0.65-0.91) and interventricular hemorrhage (IVH) (OR 2.14, 95% CI 1.05-4.35); subacute ND with 72-hour edema (OR 1.03 per mL, 95% CI 1.02-1.05) and fever (OR 2.49, 95% CI 1.01-6.14); and delayed ND with age (OR 1.11 per year, 95% CI 1.04-1.18), troponin (OR 4.30 per point, 95% CI 1.71-10.77) and infections (OR 3.69, 95% CI 1.11-12.23). Patients with ND had worse 90-day modified Rankin scores (5 vs. 3, p<0.001).
Conclusions
Neurological deterioration occurs frequently and predicts poor outcomes. Our results implicate hematoma expansion and IVH in early ND, and cerebral edema, fever, and medical complications in later ND.
Background
Neurological deterioration (ND) is a devastating complication after spontaneous intracerebral hemorrhage (ICH) that occurs in 8-33% of patients.1–4 Retrospective and registry data have shown an association between ND and large hematoma volumes (>45-60 mL), especially when mass effect and midline shift are present.3,4 While one study demonstrated ND to be associated with admission Glasgow Coma Scale (GCS) scores of <14,4 another study could not confirm this association.3
Apart from large ICH volume, which presumably drives worsening due to infarction, mass effect and brain tissue shifts, early hematoma expansion has been implicated as the cause of hyperacute ND in up to 25% of patients.3 Clinical factors associated with hematoma growth, such as elevated systolic blood pressure or presence of a spot-sign, have also been associated with ND.1,5,6
Leira et al. reported the largest prospective study examining ND after ICH.2 In their study of 261 non-comatose ICH patients presenting within 12 hours of ictus, ND occurred in 22% of patients within 48 hours of hospitalization. Admission characteristics associated with ND included IVH, temperature >37.5 C°, increased neutrophil count, and increased fibrinogen levels. Hematoma expansion and severe hypertension occurring within 48 hours of admission were also associated with ND.
ND occurs most frequently within the first 24 hours of hospital admission, and a large proportion of these cases occur within the first 6-12 hours of hemorrhage onset.3,4,7 More precise understanding of the time course and risk factors of ND during the hyper-acute stage of ICH has been lacking due to enrollment windows of prior studies that have extended from 12 to 24 hours after symptom onset.2,3 Using the Virtual International Stroke Trials Archive (VISTA) database, we studied the time course and identified radiological correlates of ND in a large cohort of ICH patients who underwent CT imaging within 3 hours of ICH onset.
Methods
Study Design and Population
We performed a retrospective cohort study of patients enrolled in the VISTA database who were enrolled in the placebo arm of prospective, randomized clinical trials of acute treatments for ICH. Our primary aim was to determine the incidence of ND at distinct time points after admission with ICH and to determine clinical and radiographic features associated with deterioration at each time point. We hypothesized that early ND would be associated with large initial ICH volumes and hematoma expansion and later ND would be associated with edema formation and intraventricular hemorrhage.
Inclusion criteria included baseline CT scan performed within 3 hours of symptom onset, follow-up CT scan at 24 and 72 hours, and GCS and NIHSS performed at baseline, 1 hour, 1 day, 2 days, 3 days, and 15 days, and available 3-month mRS score. Exclusion criteria included administration of an active investigated drug, presenting GCS of 5 or less, surgical evacuation of hematoma planned within 24 hours, secondary ICH, and known anticoagulation therapy or coagulopathy. Additional methods and exclusion criteria regarding these studies have been previously reported.8,9
Definitions
Neurological deterioration was defined as a 2 point or greater decrease in Glasgow Coma Scale (GCS) or a 4 point or greater increase in the NIHSS score. We examined the time course of neurological deterioration based on the following predefined periods based on timing of available clinical evaluations:
Hyper-acute deterioration (HD; 0-1 hours)
Acute deterioration (AD; 1-24 hours)
Sub-acute deterioration (SD; 1-3 days)
Delayed deterioration (DD; 3-15 days)
Hyperacute deterioration compared baseline and 1 hour scores. Clinical scores at other time points compared clinical scores at the beginning and end of the time period. Additionally we created two additional categories for analysis purposes: 1) No Deterioration group (NoD) who had stable or improving GCS during each period throughout the first 15 days; 2) Any Deterioration group (AnyD) consisting of all patients experiencing deterioration in any of the pre-defined time periods as well as those who experienced a gradual decline across multiple time periods compared to baseline (e.g., a 2 point NIHSS increase in the hyper-acute period and another 2 point NIHSS increase in the acute period). Given the aim of the study was to identify drivers of ND at specific time points, subjects experiencing a gradual decline in their exam were not included in a specific individual deterioration group but were included in the “Any Deterioration” group. Hematoma expansion was defined as an increase of ICH volume on follow-up CT scans of either 6 mL or >33%.
Statistical Analysis
Univariate and multivariate analyses were performed to identify demographic, clinical, and radiographic predictors of deterioration for each subgroup using Chi-Square, Fisher's Exact Test, Mann-Whitney-U, and logistic regression as appropriate. Each subgroup was compared to the pool of patients who did not experience neurological deterioration during the study (NoD group). Kaplan-Meier survival analysis was performed to test effect of clinical variables on ND-free survival. All statistical analysis was performed on commercially available software (IBM SPSS Statistics 21).
Results
Baseline Characteristics
We enrolled 376 patients with ICH into the study (Table 1). Overall, the subjects experienced mild-moderate illness, with a median GCS of 14, NIHSS of 14, and ICH volume of 14 mL. Eight patients underwent surgical hematoma evacuation that had not been planned at time of enrollment. Neurological deterioration occurred in 176 (47%) patients at any point during the study, 170 at discrete time points and 6 gradually over multiple time periods. The first episode of ND was most likely to occur within the first 24 hours (123/176, 70%), and a third of these patients deteriorated within the first hour (42/123, 34%). Compared to those with no deterioration at any point during the study, patients with hyperacute and acute ND were more likely to have lower GCS (HD, AD vs. NoD; 14, 13 vs. 15, p=0.003, p<0.001), higher NIHSS (HD, AD vs. NoD; 15, 16 vs. 12, p=0.02, p<0.001), larger hematoma volumes (HD, AD vs. NoD; 23mL, 24mL vs. 9mL, both p<0.001), and presence of IVH (HD, AD vs. NoD; 43%, 38% vs. 25%, both p=0.02). Patients with acute ND were more likely to have a lobar location of their hematoma (AD vs NoD; 24% vs. 10%, p=0.002) and had higher serum glucose (AD vs NoD; 129 vs 114, p=0.01). Patients with subacute ND shared many characteristics with hyperacute and acute ND patients, though increased rates of fever (SD vs NoD, 38% vs. 15%, p=0.003) and higher IVH blood volumes (SD vs. NoD; median 0, IQR 0-3.0mL, vs median 0, IQR 0-0, mL, p=0.006) were unique associations in this group.
Table 1.
Patient Characteristics by Neurological Deterioration Status
| Any Neurological Deterioration |
First Neurological Deterioration by Category vs. No Deterioration |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes (N=176) | No (N=200) | P | Hyperacute (HD) (N=42) | p | Acute (AD) (N=81) | p | Subacute (SD) (N=32) | P | Delayed (DD) (N=15) | p | |
| Demographics | |||||||||||
| Age (+/− StD) | 68 (+/−14) | 64 (+/−12) | 0.003 | 66 | 0.3 | 67 (+/− 15) | 0.06 | 69 (+/−12) | 0.02 | 75 (+/− 12) | 0.001 |
| Female Sex | 73 (42%) | 73 (37%) | 0.3 | 20(48%) | 0.2 | 30 (37%) | 0.9 | 14 (44%) | 0.4 | 8 (53%) | 0.3 |
| Non-White | 44 (25%) | 63 (32%) | 0.2 | 12 (29%) | 0.7 | 21 (26%) | 0.4 | 6 (19%) | 0.2 | 4 (27%) | 0.8 |
| Admission Clinical Scores | |||||||||||
| Baseline GCS | 14 (11-15) | 15 (14-15) | <0.001 | 14 (13-15) | 0.003 | 13 (11-15) | <0.001 | 14 (10-15) | 0.001 | 14 (11-15) | 0.04 |
| Baseline NIHSS | 16 (12-19) | 12 (7-16) | <0.001 | 15 (11-17) | 0.02 | 16 (13-20) | <0.001 | 17 (12-20) | 0.001 | 15 (8-20) | 0.1 |
| Admission Vitals | |||||||||||
| Systolic BP (mmHg) | 180 (160-200) | 177 (157-194) | 0.2 | 175 (160-200) | 0.8 | 180 (160-199) | 0.2 | 186 (170-207) | 0.05 | 171 (140-190) | 0.6 |
| Diastolic BP (mmHg) | 95 (80-110) | 97 (84-110) | 0.4 | 98 (80-109) | 0.5 | 95 (81-110) | 0.7 | 98 (85-110) | 0.8 | 83 (70-96) | 0.03 |
| Temperature (deg C) | 36.3 (36.0-36.6) | 36.5 (36.0-36.8) | 0.05* | 36.3 (36.2-36.6) | 0.3 | 36.4 (36.0-36.7) | 0.1 | 36.3 (36.0-36.8) | 0.3 | 36.3 (35.9-36.5) | 0.2 |
| Admission Radiographic Data | |||||||||||
| ICH Volume (ml) | 23.4 (9.9-46.6) | 9.4 (4.5-20.3) | <0.001 | 23.4 (8.7-49.2) | <0.001 | 24.4 (9.6-48.9) | <0.001 | 19.5 (12.7-47.2) | <0.001 | 15.0 (9.5-42.0) | 0.06 |
| IVH Present | 78 (44%) | 49 (25%) | <0.001 | 18 (43%) | 0.02 | 31 (38%) | 0.02 | 17 (53%) | 0.001 | 9 (60%) | 0.005 |
| IVH Volume (mL) | 0 (0-0.2) | 0 (0-0) | 0.03 | 0 (0-0) | 0.4 | 0 (0-0) | 0.2 | 0 (0-3.0) | 0.006 | 0 (0-0) | 0.7 |
| Edema Volume (mL) | 4.9 (0-21.9) | 4.1 (0-11.2) | 0.3 | 4.7 (0-14.8) | 0.8 | 6.8 (0-30.2) | 0.05* | 8.7 (0-28.7) | 0.2 | 0 (0-0.4) | 0.001 |
| Total Blood Volume (mL) | 25.7 (13.0-52.7) | 11.9 (5.0-23.4) | <0.001 | 24.9 (15.9-55.7) | <0.001 | 28.4 (12.3-54.0) | <0.001 | 23.4 (14.0-61.4) | <0.001 | 22.2 (9.6-42.0) | 0.04 |
| Lobar Location | 30 (17%) | 19 (10%) | 0.03 | 6(14%) | 0.4 | 19 (24%) | 0.002 | 3 (9%) | 1 | 1 (7%) | 1 |
| Infratentorial Location | 10 (6%) | 12 (6%) | 0.9 | 2(5%) | 1 | 6 (7%) | 0.8 | 0 (0%) | 0.4 | 2 (13%) | 0.3 |
| Past Medical History | |||||||||||
| Hypertension | 25 (14%) | 43 (22%) | 0.07 | 8(19%) | 0.8 | 10 (12%) | 0.08 | 4 (13%) | 0.3 | 1 (7%) | 0.3 |
| Diabetes | 2 (1%) | 5 (3%) | 0.4 | 0 (0%) | 0.6 | 2 (3%) | 1 | 0 (0%) | 1.0 | 0 (0%) | 1 |
| Hyperlipidemia | 18 (10%) | 26 (13%) | 0.4 | 3(7%) | 0.4 | 9 (11%) | 0.8 | 3 (9%) | 0.8 | 0 (0%) | 0.2 |
| Tobacco Use | 16 (9%) | 23 (12%) | 0.5 | 3 (7%) | 0.6 | 8 (10%) | 0.8 | 5 (16%) | 0.6 | 0 (0%) | 0.4 |
| Alcohol Use | 15 (9%) | 15 (8%) | 0.7 | 5 (12%) | 0.4 | 6 (7%) | 1 | 4 (13%) | 0.3 | 0 (0%) | 0.6 |
| Admission Laboratory Values | |||||||||||
| Glucose (mg/dL) | 124 (105-158) | 114 (99-145) | 0.04 | 124 (103-159) | 0.4 | 129 (105-160) | 0.01 | 121 (106-160) | 0.2 | 112 (95-124) | 0.3 |
| Creatinine (mg/dL) | 1.0 (0.8-1.3) | 1.0 (0.8-1.3) | 0.7 | 0.9 (0.8-1.2) | 0.8 | 1.0 (0.8-1.2) | 0.7 | 1.0 (0.8-1.3) | 0.8 | 0.9 (0.8-1.3) | 0.8 |
| Sodium (mmol/L) | 140 (138-142) | 140 (138-142) | 0.6 | 139 (137-141) | 0.3 | 140 (138-142) | 1 | 140 (138-142) | 1 | 139 (138-142) | 0.8 |
| INR | 1.0 (0.9-1.2) | 1.0 (0.9-1.1) | 0.5 | 1.0 (0.8-1.2) | 0.8 | 1.0 (0.9-1.2) | 0.3 | 1.0 (0.9-1.1) | 0.9 | 1.0 (0.9-1.2) | 0.8 |
| aPTT (sec) | 29.0 (26.0-33.3) | 28.0 (26.2-31.1) | 0.2 | 29.0 (26.3-33.0) | 0.4 | 27.8 (25.3-32.2) | 0.9 | 29.9 (26.4-34.0) | 0.1 | 31.2 (27.9-36.4) | 0.1 |
| Troponin (ug/L) | 0.1 (0.05-0.25) | 0.05 (0.05-0.10) | 0.002 | 0.1 (0.05-0.25) | 0.05 | 0.05 (0.05-0.25) | 0.04 | 0.05 (0.05-0.25) | 0.3 | 0.3 (0.1-0.3) | 0.001 |
| Cholesterol (mmol/L) | 5.0 (4.0-5.6) | 5.1 (4.2-5.8) | 0.3 | 4.7 (3.9-5.6) | 0.5 | 4.8 (3.8-5.4) | 0.1 | 5.4 (4.3-6.0) | 0.3 | 5.2 (4.3-5.3) | 0.7 |
| Fibrin (g/L) | 4.0 (3.1-4.8) | 3.8 (3.0-4.6) | 0.2 | 3.5 (2.6-4.3) | 0.3 | 3.8 (3.1-4.8) | 0.4 | 4.2 (3.5-5.2) | 0.02 | 4.7 (3.3-5.1) | 0.03 |
| Factor 1+2 (nmol/L) | 1.0 (0.6-1.5) | 0.7 (0.4-1.2) | 0.001 | 0.9 (0.5-1.4) | 0.2 | 1.1 (0.5-1.5) | 0.004 | 0.7 (0.5-1.3) | 0.3 | 1.3 (0.9-2.5) | 0.001 |
Categorical Variables are noted as N (%). Continuous variables are as noted.
Continuous variables are in the units noted followed by either +/− standard deviation or interquartile range.
Non-significant p-value before rounding
Neurological Deterioration-Free Survival Curves
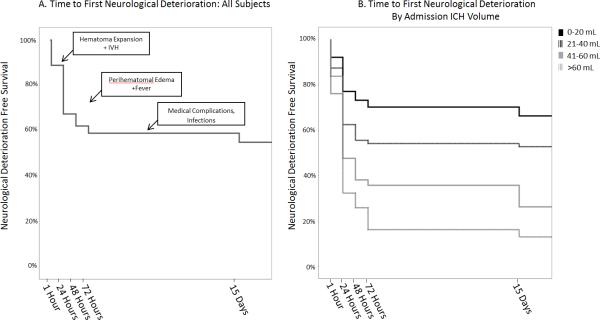
Figure 1 demonstrates a Kaplan-Meier curve of ND-free survival in all patients (Figure 1A) and by admission ICH-volume strata (Figure 1B). Of those patients who did not experience ND by 72 hours, only 6% went on to have delayed ND by day 15. For those with ND in the first 72 hours, 22% had further delayed ND.
Figure 1.
ND-Free Survival. A) All Patients B) Stratified by ICH Volume
CT Characteristics
Characteristics of baseline, 24 hour, and 72 hour CT scans are reported in Table 2. Both HD and AD were associated with hematoma expansion from baseline to 24 hour scans compared to the NoD group (HD, AD vs. NoD; 46%, 63% vs. 20%; both p<0.001). Patients with ND in the first 24 hours had higher volumes of hematoma expansion (11mL vs 1mL, p<0.001). Hematoma expansion was not associated with subacute ND. Although median IVH volumes were 0 at baseline and 24 hours in both groups, there was a significant increase in IVH volumes in those with ND in the first 24 hours. Increased IVH volumes on 24 hour and 72 hours scans were associated with subacute deterioration. Edema volume was not associated with ND in the first 24 hours, but volume of edema at 72 hours (43 vs 18 mL, p<0.001) were associated with subacute ND.
Table 2.
CT Dynamics
| Hyperacute or Acute ND |
Subacute ND |
||||||
|---|---|---|---|---|---|---|---|
| Yes | No | p | Yes | No | p | ||
| ICH Volume | (Compares Baseline to 24H Scans) | ICH Volume | (Compares Day 1 to Day 3 Scans) | ||||
| Admission Scan Volume (mL) | 24 (9-49) | 9 (5-20) | <0.001 | 24-Hour Scan Volume (mL) | 33 (14-53) | 11 (5-24) | <0.001 |
| 24-Hour Scan Volume (mL) | 37 (12-70) | 11 (5-24) | <0.001 | 72-Hour Scan Volume (mL) | 33 (12-51) | 10 (4-23) | <0.001 |
| Median Change (mL) | 11 (0-24) | 1 (0-2) | <0.001 | Median Change (mL) | 0 (−4-1) | 0 (−1-0) | 0.5 |
| IVH Volume | IVH Volume | ||||||
| Admission Scan Volume (mL) | 0 (0-0) | 0 (0-0) | 0.1 | 24-Hour Scan Volume (mL) | 0 (0-4) | 0 (0-0) | 0.006 |
| 24-Hour Scan Volume (mL) | 0 (0-5) | 0 (0-0) | <0.001 | 72-Hour Scan Volume (mL) | 0 (0-3) | 0 (0-0) | 0.003 |
| Median Change (mL) | 0 (0-4) | 0 (0-0) | <0.001 | Median Change (mL) | 0 (−1-0) | 0 (0-0) | 0.2 |
| Edema Volume | Edema Volume | ||||||
| Admission Scan Volume (mL) | 6 (0-22) | 4 (0-11) | 0.2 | 24-Hour Scan Volume (mL) | 24 (0-53) | 10 (3-22) | 0.07 |
| 24-Hour Scan Volume (mL) | 12 (0-51) | 10 (3-22) | 0.1 | 72-Hour Scan Volume (mL) | 43 (18-86) | 18 (10-35) | <0.001 |
| Median Change (mL) | 4 (0-27) | 3 (0-9) | 0.1 | Median Change (mL) | 14 (1-40) | 5 (1-14) | 0.03 |
All volumes are in mL and presented as medians (IQR).
Multivariable Model for ND
In multivariate analyses of ND within predefined categories, hyperacute ND was associated with hematoma expansion (OR 3.6, 95% CI 1.7-7.6) and baseline ICH volume (OR 1.04 per mL, 95% CI 1.02-1.06); acute ND with hematoma expansion (OR 7.59, 95% CI 3.91-14.74), baseline ICH volume (OR 1.02 per mL, 95% CI 1.01-1.04), admission GCS (OR 0.0.77 per point, 95% CI 0.65-0.91) and presence of IVH (OR 2.14, 95% CI 1.05-4.35); subacute ND with edema volume at 72 hours (OR 1.03 per mL, 95% CI 1.02-1.05) and fever (OR 2.49, 95% CI 1.01-6.14); and delayed ND with age (OR 1.11 per year, 95% CI 1.04-1.18) troponin levels (OR 4.30 per point, 95% CI 1.71-10.77) and infection in the 3 to 15 day period (OR 3.69, 95% CI 1.11-12.23)
Outcomes
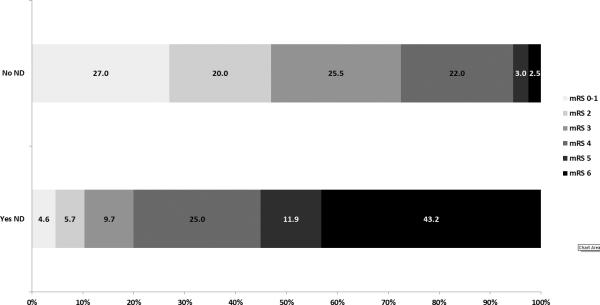
Three month outcomes are listed in Table 3. Patients with ND at any point in the study were more likely to die (42% vs 3%, p<0.001) and have lower median mRS (5 vs. 3, p<0.001). Of the five patients who died between day 15 and 90, three died from medical complications, one from a fall, and another due to progression of neurological deficits. Breakdown of mRS stratifying for ND can be seen in Figure 2.
Table 3.
Outcomes
| Neurological Worsening in Study |
|||
|---|---|---|---|
| Yes | No | p | |
| 90 –Day Outcomes | |||
| Dead | 74 (42%) | 5 (3%) | <0.001 |
| mRS | 5 (4-6) | 3 (1-4) | <0.001 |
| Barthel | 8 (0-50) | 90 (65-100) | <0.001 |
| NIHSS | 14 (6-32) | 3 (1-6) | <0.001 |
| European QOL | 0.12 (0.00-0.71) | 0.76 (0.42-0.83) | <0.001 |
Figure 2.
90 Day Outcomes by ND Status
Discussion
We found that when the initial clinical evaluation is performed within 3 hours of onset, ND after ICH is very common: almost half of patients will clinically worsen at some point during their first 15 days of hospitalization. Interestingly, deterioration is not a complication reserved just for patients with very large bleeds or very poor clinical presentations. The median admission GCS for those experiencing ND at any point in the first 15 days was 14 and median ICH size was 23 mL. We also found a striking association between ND and poor outcome. Patients who deteriorate are more likely to have further worsening during their hospitalization, and mortality occurs almost exclusively among patients who have worsened. Clinical strategies focused on prevention of ND are a logical step for improving outcome after ICH.
What are the drivers leading patients to worsen at different points during their hospitalization? We confirmed prior studies demonstrating that ND within 24 hours of onset is driven primarily by the amount of blood, including acute hematoma expansion. We also found that IVH is a significant driver of acute deterioration, likely due to multiple factors including neurotoxicity of blood products, especially on the diencephalon, and hydrocephalus. Patients who worsened between 24 and 72 hours had large edema volumes and subacute fevers, pointing to these entities as drivers of deterioration during this time period. Delayed worsening between days 3 and 15 was seen in patients with increased age, troponin levels, and infectious complications, suggesting that medical complications and lack of functional reserve may be driving delayed deterioration. However, causation in this time period is difficult to assess as medical complications could also arise as a result of long hospital stays in those with neurological deterioration.
There are weaknesses to this study. Most importantly, patients in this study needed to meet stringent enrollment criteria for participation in Phase II and III randomized-controlled trials and had to consent to participation. Therefore, our study population is biased toward less disease severity than the ICH population at large. There was not a standardized way to account for residual effect of anesthetic agents in follow-up clinical assessments in the eight patients who underwent hematoma evacuation. While they are included in the final analysis, their exclusion did not meaningfully change the multivariate models. Additionally, while we defined the hyperacute period as being the first hour after enrollment, our inclusion criteria allowed enrollment of patients for up to three hours post-ictus. Thus, the exact post-ictus timing of the hyperacute period varied depending on when the patient presented and was enrolled. There are also considerable strengths to this study. It is multi-center, with prospective data collection at pre-established time points, and with five clinical assessments and three CTs within the first 72 hours.
Our study has clinical implications. In practice, we should prepare families—who often expect patients to improve rather than worsen after hospitalization—that chances are equally likely for worsening as they are for stability or improvement. This is true for patients with even moderate size hemorrhages and decent initial clinical exams. Conversely, lack of deterioration is a very favorable finding and implies a favorable long term prognosis.
Neurological deterioration remains a common and devastating complication after ICH. While some risk factors are not modifiable, others, including hematoma expansion in the first 24 hours and edema volume and fever in the subacute period, are amenable to treatment. Given the deleterious impact on outcomes, we should continue to search for treatments which prevent hematoma expansion, edema formation, and fever in order to reduce the impact of ND on outcomes. Further trials focused on arresting early hematoma growth with hemostatic therapy are justified, as are trials of medical and surgical interventions for reducing compartmentalized mass effect and tissue shifts.
Acknowledgments
Funding:
Dr. Lord received support from the New York University-Health and Hospitals Corporation Clinical and Translational Science Institute via grant UL1 TR000038 from the National Center for Advancing Translational Sciences of the National Institutes of Health.
Footnotes
Disclosures:
None.
APPENDIX
VISTA-ICH Steering Committee
D. F. Hanley (Chair), K. Butcher, S. Davis, B. Gregson, K.R. Lees, P. Lyden, S. Mayer, K. Muir and T. Steiner.
References
- 1.Sakamoto Y, Koga M, Yamagami H, Okuda S, Okada Y, Kimura K, et al. Systolic Blood Pressure After Intravenous Antihypertensive Treatment and Clinical Outcomes in Hyperacute Intracerebral Hemorrhage: The Stroke Acute Management With Urgent Risk-Factor Assessment and Improvement-Intracerebral Hemorrhage Study. Stroke. 2013;44:1846–1851. doi: 10.1161/STROKEAHA.113.001212. [DOI] [PubMed] [Google Scholar]
- 2.Leira R, Davalos A, Silva Y, Gil-Peralta A, Tejada J, Garcia M, et al. Early neurologic deterioration in intracerebral hemorrhage: Predictors and associated factors. Neurology. 2004;63:461–467. doi: 10.1212/01.wnl.0000133204.81153.ac. [DOI] [PubMed] [Google Scholar]
- 3.Mayer SA, Sacco RL, Shi T, Mohr JP. Neurologic deterioration in noncomatose patients with supratentorial intracerebral hemorrhage. Neurology. 1994;44:1379–1384. doi: 10.1212/wnl.44.8.1379. [DOI] [PubMed] [Google Scholar]
- 4.Flemming KD, Wijdicks EF, St Louis EK, Li H. Predicting deterioration in patients with lobar haemorrhages. J Neurol Neurosurg Psychiatry. 1999;66:600–605. doi: 10.1136/jnnp.66.5.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez-Luna D, Pineiro S, Rubiera M, Ribo M, Coscojuela P, Pagola J, et al. Impact of blood pressure changes and course on hematoma growth in acute intracerebral hemorrhage. Eur J Neurol. 2013;20:1277–83. doi: 10.1111/ene.12180. [DOI] [PubMed] [Google Scholar]
- 6.Sorimachi T, Osada T, Baba T, Inoue G, Atsumi H, Ishizaka H, et al. The striate artery, hematoma, and spot sign on coronal images of computed tomography angiography in putaminal intracerebral hemorrhage. Stroke. 2013;44:1830–1832. doi: 10.1161/STROKEAHA.113.001498. [DOI] [PubMed] [Google Scholar]
- 7.Britton M, Roden A. Progression of stroke after arrival at hospital. Stroke. 1985;16:629–632. doi: 10.1161/01.str.16.4.629. [DOI] [PubMed] [Google Scholar]
- 8.Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–2137. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
- 9.Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352:777–785. doi: 10.1056/NEJMoa042991. [DOI] [PubMed] [Google Scholar]