Figure 6.
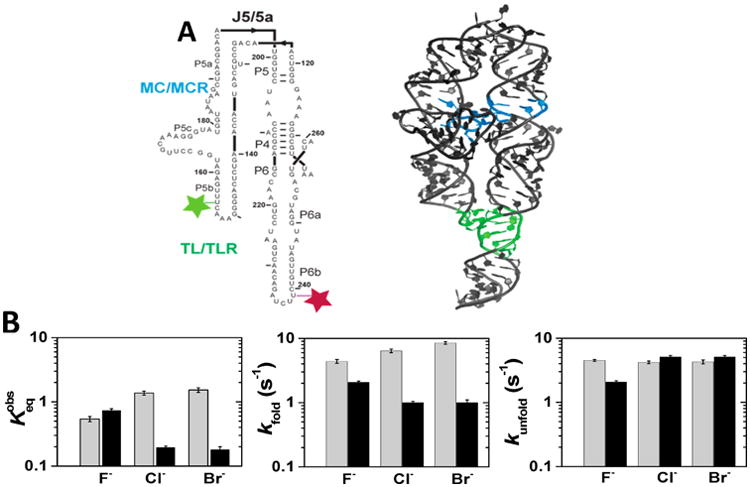
P4–P6 folding kinetics and thermodynamics as a function of the cation and anion identity. (A) Secondary (left) and crystallographic (right) structure of the P4–P6 domain of the Tetrahymena group I intron. Tertiary contacts are colored as follows: the tetraloop/tetraloop receptor TL/TLR (green), and the metal core/metal core MC/MCR receptor (blue). Dye placements used for smFRET are shown: Cy3 (light green) and Cy5 (maroon).62,65 The P4–P6 crystallographic structure has both tertiary contacts formed, but the experiments herein were carried out in the absence of Mg2+, and thus the MC/MCR is not formed.80 (B) Folding and unfolding rate constants and the equilibrium constant for P4–P6 RNA folding at 1 M NaX (in gray) and 1 M RbX (in black). The folding equilibrium is defined as the ratio of the folding rate constant to unfolding rate constant: . Error bars correspond to the bootstrap-estimated 95% confidence intervals (SD = 2σ bootstrap).
