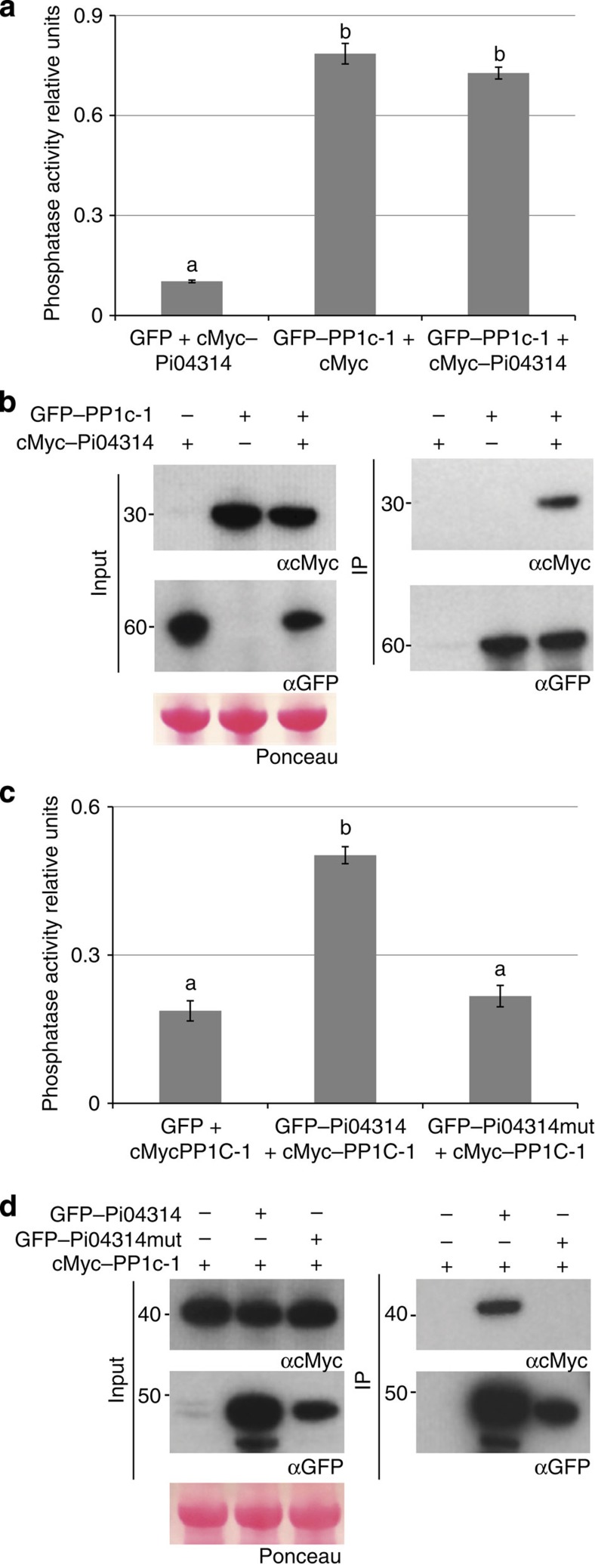
Figure 7. Pi04314 does not inhibit PP1c-1 phosphatase activity.
(a) Graph of phosphatase activity measured directly on GFP–Trap_M beads following immunoprecipitation of GFP co-expressed with cMyc–Pi04314; or GFP–PP1c-1 co-expressed with either cMyc vector or cMyc–Pi04314. Error bars are s.e. and the graph represents combined data from three biological replicates. Letters on the graph denote statistically significant differences (ANOVA, P<0.001). (b) Immunoprecipitation of protein extracts from agroinfiltrated leaves using GFP–Trap confirmed that cMyc–Pi04314 is co-immunoprecipitated with GFP–PP1c-1. Expression of constructs in the leaves is indicated by +. Protein size markers are indicated in kDa, and protein loading is indicated by Ponceau stain. Antibodies used are indicated (αcMyc and αGFP). (c) Graph of phosphatase activity measured directly on GFP–Trap_M beads following immunoprecipitation of GFP, GFP–Pi04314 or GFP–Pi04314mut co-expressed with cMyc–PP1c-1. Error bars are s.e. and the graph represents the combined data from three biological replicates. Letters on the graph denote statistically significant differences (ANOVA, P<0.001). (d) Immunoprecipitation of protein extracts from agroinfiltrated leaves using GFP–Trap confirmed the cMyc–PP1c-1 co-immunoprecipitated with the GFP–Pi04314 effector, but not with GFP–Pi04314mut. Expression of constructs in the leaves is indicated by +. Protein size markers are indicated in kDa, and protein loading is indicated by Ponceau stain. Antibodies used are as indicated (αcMyc and αGFP).