Abstract
Objective
The new CDC paradigm for ventilator-associated events (VAE) is intended to simplify surveillance of infectious and non-infectious complications of mechanical ventilation in adults. We assessed the VAE algorithm in pediatric patients.
Design
A retrospective observational cohort study.
Setting
This single center study took place in a PICU at an urban academic medical facility.
Patients
Pediatric (ages 0–18) trauma patients with moderate-to-severe traumatic brain injury (TBI) ventilated for ≥2 days.
Measurements and Main Results
We assessed for pediatric ventilator-associated pneumonia (VAP; as defined by current CDC PNU2 guidelines), adult VAE, and an experimental VAE definition modified for pediatric patients. We compared VAE to VAP to calculate test characteristics. Thirty-nine (33%) of 119 patients developed VAP. Sensitivity of the adult ventilator-associated condition (VAC) definition was 23% (95% CI 11–39%), which increased to 56% (95% CI 40–72%) using the modified pediatric (VAPMP) criterion. Specificity reached 100% for both original and MP probable VAP using VAE criteria. Children who developed VAP or VAC had similar baseline characteristics: age, mechanism of injury, injury severity scores and use of an intracranial pressure monitor. Diagnosis of VAP and VAC portended similarly unfavorable outcomes: longer median duration of ventilation, ICU and hospital length of stay, and more discharges to rehab, home health or nursing care compared to patients with no pulmonary complication.
Conclusions
Both current and modified VAE criteria have poor sensitivity but good specificity in identifying pediatric VAP. Despite poor sensitivity, the high specificity of the VAE diagnoses does provide a useful and objective metric for inter-institution ICU comparison. VAP and VAC were both associated with excess morbidity in pediatric TBI patients.
Keywords: ventilator-associated pneumonia, PNU2, ventilator-associated condition, ventilator-associated event, pediatric, traumatic brain injury
Introduction
Healthcare-associated infections are a patient safety concern and are increasingly included as care quality metrics.(1–3) Pulmonary infections related to mechanical ventilation (ventilator-associated pneumonia or VAP) are common in intensive care units managing patients of all ages(4, 5) and are associated with substantial increases in cost, length of stay, morbidity, and mortality.(4, 6–9) Among pediatric patients suffering trauma, those with brain injuries are particularly susceptible to VAP.(7, 10, 11)
The challenge facing investigators and clinicians attempting to study, diagnose, and treat VAP remains establishment of a “gold standard” definition. Current pediatric VAP criteria (PNU2)(12) rely on subjective data such as radiographs and clinical signs which demonstrate poor sensitivity and specificity.(13, 14) Inter-institution surveillance has proven difficult in the setting of variable interpretation and manipulation of present definitions.(14–17) A more objective algorithm was recently developed and endorsed by the Centers for Disease Control (CDC) for use in adult patients with the intent of improving surveillance for clinically significant ventilator-associated pneumonia and non-infectious ventilator complications.(18–22) The updated criteria focus on respiratory deterioration due to a spectrum of ventilator-associated events (VAE): ventilator-associated condition (VAC), infection-related ventilator-associated complication (IVAC), possible pneumonia and probable pneumonia. Respiratory deterioration is defined by measures of decreased oxygenation reflected in ventilator settings (i.e. increased positive end-expiratory pressure (PEEP) and/or fraction of inspired oxygen (FiO2)). The new criteria defining VAE-VAP do not include potentially subjective interpretations of radiographic or clinical findings. In the original adult validation study, the VAE algorithm accurately predicted commonly reported outcomes (length of ventilation, length of hospital stay) more effectively than traditional VAP diagnostic criteria, and overall the strategy took less time to implement for retrospective surveillance.(20)
The adult VAE criteria have not been validated in infants and children and no pediatric specific parameters have been proposed. As in adults, a consistent, objective and reliable pediatric definition of ventilator-associated pneumonia is required if this diagnosis is to be used in benchmarking quality between medical institutions.
We chose a cohort of pediatric patients with traumatic brain injury (TBI), who have comparably higher risk of ventilator-associated complications than other critically ill children, to test the adult VAE criteria. We developed modified pediatric (MP) criteria using the same conceptual framework and data elements as the adult diagnostic algorithm. While pulmonary mechanics of ventilation are similar in adult and pediatric patients, common ventilator management strategies may differ. Consequently, the proposed changes in PEEP and FiO2 may not be sensitive or specific when surveying a pediatric cohort for respiratory complications.(23) Using the current CDC ventilator-associated pneumonia definition that requires a positive microbiologic specimen (PNU2)(12) as the gold standard, we calculated and compared test characteristics of both the original and our modified pediatric criteria.
Materials and Methods
Setting, patients, and ethics review
This retrospective cohort study evaluated pediatric trauma patients treated at an American College of Surgeons verified, level I pediatric trauma center within a large academic facility in Salt Lake City, UT. We reviewed patients (0–18 years old) admitted to the pediatric intensive care unit (PICU) from 2009 to 2012 with moderate-to-severe traumatic brain injury (TBI; GCS<13) who received mechanical ventilation for ≥2 days after injury. Patients were excluded if they died or developed pneumonia ≤48 hours after admission, or had penetrating brain injury.
The institutional review board at the University of Utah and privacy board for Primary Children’s Hospital reviewed the study and waived need for informed consent as the study involved retrospective chart review and did not impact patient care.
Data collection
Patient demographics, mechanism of injury, injury severity scores, and outcome data were obtained from the available trauma database, nurse charting and electronic records. Emergency medical service and emergency room documentation was utilized to identify the post-resuscitation Glasgow Coma Score (GCS). Hourly respiratory charting was reviewed and the minimum PEEP and FiO2 extracted for each day of hospitalization. Chest radiographs at the time of respiratory decompensation were evaluated by 2 independent investigators (MH, MC) and discordant findings adjudicated by a third (SB).
Definitions
Ventilator-associated pneumonia (VAP)
The CDC PNU2 definition is outlined in the PNEU/VAP device-associated module.(12) This definition requires presence of a persistent imaging abnormality, 2 signs or symptoms of pneumonia and a positive microbiologic specimen meeting CDC threshold guidelines.(22) Protected bronchial brushing was the primary method of sampling (98%) in this study. Patients were censored from analysis two days post-extubation, at the time of tracheostomy or death. In this study, those patients with no microbiologic specimen were presumed negative for clinical VAP.
Ventilator-associated Events (VAE)
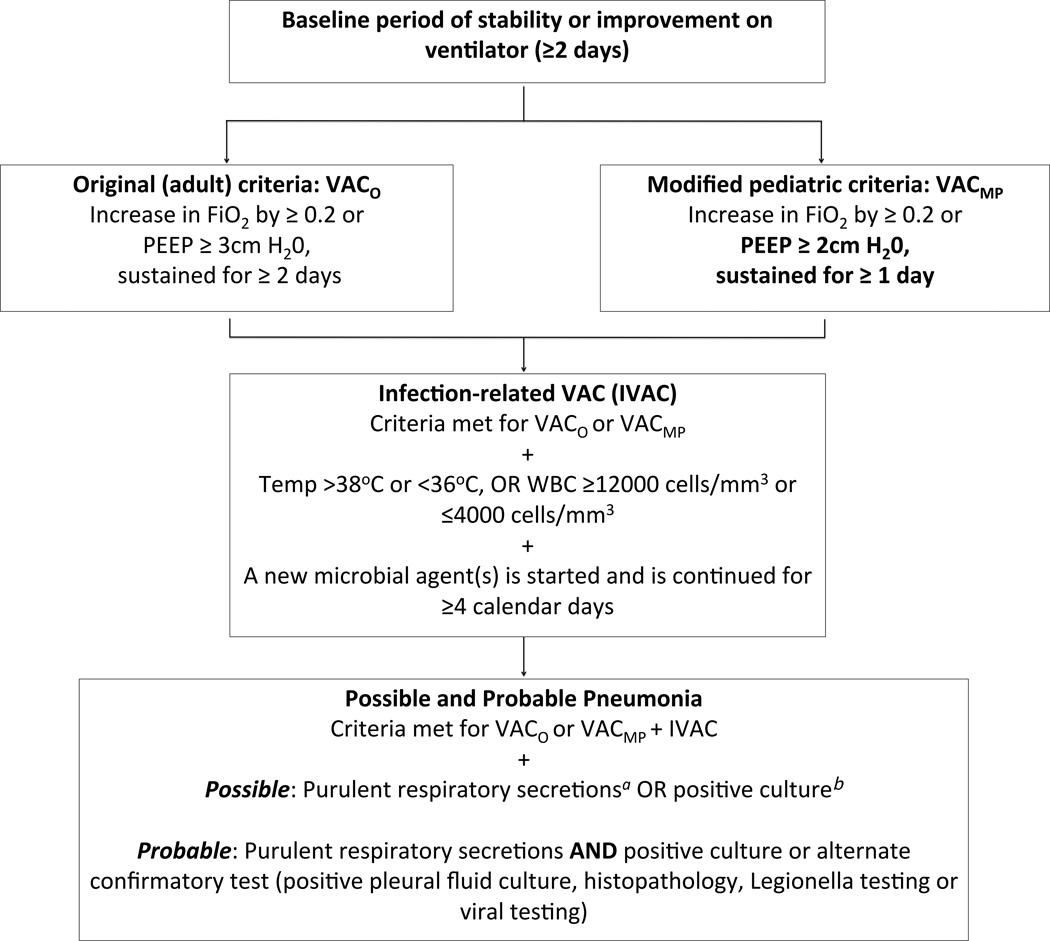
The current adult definitions for ventilator-associated condition (VAC), infectious ventilator-associated condition (IVAC), possible and probable pneumonia are outlined in Figure 1.(22, 24) Patients whose ventilator settings met the original National Healthcare Safety Network (NHSN) VAC criteria are delineated as VACO. Prior to data collection and analysis, we devised experimental “modified pediatric” parameters for VAC under guidance of local intensivists (VACMP or MP) (Figure 1). VACO and VACMP, IVAC, possible and probable pneumonia were identified manually and using the statistical software program R 3.1.3 (R Foundation for Statistical Computing, Vienna, Austria).
Figure 1.
The VAE Algorthim, including the original adult VAC criteria (VACO) and the modified pediatric (VACMP) criteria.
VAC=ventilator-associated condition; PEEP=Positive end expiratory pressure; FiO2= Fraction of inspired oxygen; aPurulent secretions defined as ≥25 polymorphonuclear cells (PMNs), ≤10 squamous epithelial cells per low-powered field (LPF); bPositive culture= endotracheal aspirate ≥105 colony forming units (CFU)/ml, bronchoalveolar lavage ≥104 CFU/ml or protected brush specimen ≥103 CFU/ml (or corresponding semi-quantitative result).
Statistical analysis
Analysis was performed with SPSS version 22.0 (IBM Corporation, Armonk, New York, United States). The test characteristics of the original adult and modified pediatric VAE were assessed using VAP as the comparison diagnosis. Cohort characteristics and outcomes were compared between the overlapping pulmonary diagnosis groups and between those patients with and without any pulmonary dysfunction (negative for VAP and VAE). All statistical comparisons were standard two-sided non-parametric tests with alpha ≤0.05.
Results
Demographic and clinical features of the 119 eligible subjects are reported in Table 1, along with a comparison between those with VAP, VAC and no pulmonary diagnosis. Ventilator-associated pneumonia (VAP) was diagnosed in 39 patients (33%). Compared to those with no pulmonary diagnosis, subjects with VAP were somewhat older (median age 9 vs. 6 years; p=0.04) and more severely injured (ISS>20: 95% vs. 77%; p=0.01). Children with VAP were less often injured by non-accidental trauma (8% vs. 25%; p=0.03) and more likely to have an ICP monitor (92% vs. 41%; p<0.001; fiberoptic or external ventricular drain). Comparing patients with no pulmonary diagnosis (n=68) to those with either VAC group or VAP (n=51) showed consistent significant differences in injury characteristics such as greater rates of NAT, lower ISS and lower use of ICP monitors (Table 1). VAC and VAP groups, on the other hand, were similar.
Table 1.
A comparison of the demographic and clinical features of all patients, those with no pulmonary diagnosis (negative for VAP and VAC), those with VAP and those who met the original (VACO) or the modified pediatric (VACMP) criteria.
| Characteristics (n,%) |
All (n=119) |
No pulmonary diagnosis (n=68) |
VAP (n=39) |
VACO positive (n=12) |
VACMP positive (n=34) |
|---|---|---|---|---|---|
| Female | 34 (29) | 18 (27) | 12 (31) | 4 (33) | 10 (29) |
| Median age in years (IQR) | 8 (2–13) | 6 (2–12)* | 9 (6–13) | 8 (4–14) | 11 (5–14) |
| Race | |||||
| White | 91 (76) | 48 (71) | 36 (92) | 9 (75) | 28 (82) |
| Mechanism | |||||
| Motorized trauma | 70 (59) | 38 (56) | 24 (62) | 8 (67) | 22 (65) |
| Non-motorized trauma | 28 (24) | 13 (19) | 12 (31) | 3 (25) | 10 (29) |
| Non-accidental trauma | 21 (18) | 17 (25)* | 3 (8) | 1 (8) | 2 (6) |
| Injury severity | |||||
| Cardiac arrest prior to admission | 12 (10) | 8 (12) | 4 (10) | 1 (8) | 3 (9) |
| Median ISS (IQR) | 29 (25–35) | 27 (21–35) | 29 (25–38) | 31 (25–49) | 30 (25–37) |
| ISS >20 | 100 (84) | 52 (77)* | 37 (95) | 12 (100) | 32 (94) |
| Any chest AIS >3 | 12 (10) | 10 (15) | 3 (8) | 1 (8) | 4 (12) |
| Severe TBI (GCS <9) | 95 (80) | 52 (77) | 31 (80) | 9 (75) | 29 (85) |
| Median Rotterdam Score (IQR) | 3 (2–4) | 3 (2–4) | 3 (2–4) | 2 (2–3) | 3 (2–4) |
| Any ICP monitor | 71 (60) | 28 (41)* | 36 (92) | 9 (75) | 27 (79) |
p<0.05 comparing no pulmonary diagnosis group to combined pulmonary diagnosis group.
IQR=Interquartile range; VAP=PNU2 ventilator-associated pneumonia; VAC=ventilator-associated condition; ISS=injury severity score; AIS=abbreviated injury scale; TBI=traumatic brain injury; GCS=Glasgow Coma Score; ICP=intracranial pressure.
Table 2 reports the sensitivity, specificity and positive likelihood ratio (LR) of the original and proposed modified pediatric VAE subgroups in comparison to VAP. Nine of 39 VAP patients met criteria for VACO, and 22 for VACMP, corresponding to an increase in sensitivity from 23% to 56% when using the modified pediatric algorithm. Decreased specificity in the VACMP group resulted in a comparably lower positive likelihood ratio (3.8 vs. 6.2). Neither the original or modified pediatric VAE VAP diagnoses were sensitive in detecting VAP, but they exhibited specificity approaching 100% with progression through the algorithm.
Table 2.
Test characteristics of the original adult VAE and modified pediatric VAE criteria when compared to current CDC VAP (PNU2).
| Sensitivity (95% CI) | Specificity (95% CI) | Positive LR (95% CI) | |
|---|---|---|---|
| VACO | 23% (11–39%) | 96% (89–99%) | 6.2(1.8–21.4) |
| IVACO | 21% (9–36%) | 96% (89–99%) | 5.5(1.5–19.5) |
| Possible VAPO | 21% (9–36%) | 99%(93–100%) | 16.4 (2.1–126.6) |
| Probable VAPO | 18% (8–34%) | 100% (95–100%) | ∞ |
| Modified Pediatric | |||
| VACMP | 56% (40–72%) | 85% (75–92%) | 3.8 (2.1–6.8) |
| IVACMP | 46% (30–63%) | 94% (86–98%) | 7.4(3.0–18.4) |
| Possible VAPMP | 46% (30–63%) | 99% (93–100%) | 36.9 (4.1–266.6) |
| Probable VAPMP | 33% (19–50%) | 100% (96–100%) | ∞ |
VAC=ventilator-associated condition; IVAC=infectious-ventilator associated condition; LR=likelihood ratio.
Three patients not diagnosed with VAP met VACO criteria (3/80; 4%), and 12 met VACMP (12/80; 15%). The radiographic diagnosis and final VAE definition are outlined in Table 3. No patients met probable VAP as they either did not have a specimen collected or the specimen was culture negative. Only 58% of modified pediatric VAC cases had a microbiologic specimen obtained. Fifty percent met criteria for IVAC or possible pneumonia.
Table 3.
Cause of respiratory deterioration in cases meeting original or modified VAC diagnostic criteria (VACO or VACMP) but negative for VAP.
| Specimen obtaineda |
Reported radiographic finding at time of respiratory deterioration and VAE diagnosis |
Final VAE Diagnosis |
|---|---|---|
| VACOpositive/cVAP negative (n=3)b | ||
| Yes | Atelectasis/multi-lobar collapse | IVACO/MP |
| Yes | Atelectasis/multi-lobar collapse | IVACO/MP |
| Yes | Atelectasis/infiltrate | Possible VAPO/MP |
| VACMPpositive/cVAP negative (n=12) | ||
| No | No clear radiographic causec | VACMP |
| No | Atelectasis | VACMP |
| No | Infiltrate | IVACMP |
| Yes | Atelectasis | VACMP |
| Yes | Pleural effusion | VACMP |
| Yes | Pleural effusion | Possible VAPMP |
| Yes | Atelectasis | VACMP |
| Yes | Pulmonary contusion | VACMP |
| No | Atelectasis/pulmonary contusion | IVACMP |
Specimen obtained=Microbiologic specimen collected from patient, largely protected bronchial brush specimen (PBB; see text); None of the collected specimens in this cohort grew pathogenic bacteria positive by CDC thresholds; see Table 1 or Figure 2 legend for diagnostic abbreviations;
all VACO positive/cVAP negative cases also met criteria for VACMP;
Patient 4 had refractory intracranial hypertension and multi-organ failure with increased PEEP to improve tissue oxygenation.
Support was ultimately withdrawn 24 hours after the patient met VACMP criteria.
Table 4 displays outcomes of subjects without a pulmonary diagnosis in comparison to those with VAP, VACO or VACMP. Those patients with a pulmonary complication (VAP or VAC) had similarly poor outcomes, specifically higher rates of tracheostomy, prolonged mechanical ventilation, PICU and overall hospital length of stay, compared to patients without a pulmonary diagnosis. Although not statistically significant, patients without a pulmonary condition had a higher rate of death before discharge than the combined pulmonary diagnosis group (p=0.1), but those that survived were substantially more likely to be discharged home (p=0.005) without rehabilitation or long-term nursing care.
Table 4.
A comparison of outcomes between patients with orwithout a pulmonary diagnosis (VAP, VACO and VACMP).
| Outcomes (Median, IQR) |
No pulmonary diagnosis (n=68) |
VAP Positive (n=39) |
VACO Positive (n=12) |
VACMP Positive (n=34) |
|---|---|---|---|---|
| Days of mechanical ventilation | 4 (2–5)* | 9 (7–12) | 9 (8–47) | 10 (8–13) |
| Length of ICU stay in days | 5 (3–7)* | 12 (8–14) | 11 (10–22) | 12 (9–14) |
| Length of hospitalization in days | 11 (6–17)* | 21 (16–23) | 22 (17–53) | 20 (14–23) |
| Tracheostomy (n(%)) | 3 (4)^ | 7 (18) | 2 (17) | 6 (18) |
| Discharge status of survivors (n(%))^ | ||||
| Home | 29 (43) | 9 (23) | 2 (17) | 7 (21) |
| Home health, rehabor long-term nursing care | 23 (34) | 27 (70) | 10 (83) | 21 (62) |
| Death before discharge | 16 (24) | 3 (8) | 0 | 6 (18) |
p<0.001 or
p<0.05 comparing no pulmonary diagnosis group to combined pulmonary diagnosis group; see Table 1 or Figure 2 legends for diagnostic abbreviations.
Discussion
Among children requiring mechanical ventilation for ≥2 days after traumatic brain injury, VAP was identified in 39 of 119 patients (33%), similar to reported rates in other TBI cohorts.(25–27) Subjects diagnosed with VAP, VACO or VACMP had comparable clinical characteristics. Comparison between VAP and the original adult criteria (VACO) demonstrates high specificity for possible or probable VAP, but poor sensitivity throughout the diagnostic algorithm. A modified pediatric (VACMP) definition of respiratory compromise improved case capture, but the sensitivity remained too low for the surveillance criteria to be a useful clinical tool in their current form, and lower specificity compromised the positive LR of a VAP.(28) The presence of a pulmonary diagnosis (VAP or VAC) was associated with substantially worse clinical outcomes compared to the group without any respiratory complications.
Patients with VAP had similar severity of TBI (Glasgow Coma and Rotterdam scores) as those with no pulmonary diagnosis, with the exception of ICP monitors, which, similar to other studies, were present more frequently in those who developed VAP.(29–31) Prior investigations have shown that interventions for managing elevated intracranial pressure may independently increase risk of hospital-acquired infection in trauma patients.(29) Patients with a pulmonary condition were slightly older and less likely victims of non-accidental trauma (NAT). Younger children are more likely to suffer NAT(32), which may explain the age discordance between groups, and VAP is less likely to occur in NAT compared with other types of brain trauma.(29, 33)
VACO identified 23% of VAP cases, slightly lower than similar adult studies that reported sensitivity of 26–33%.(28, 34, 35) The modified pediatric (VACMP) definition increased sensitivity to 56%. With both the VACO and VACMP criteria, sensitivity decreased with progression through the VAE algorithm, with only 18% (original) and 33% (MP) of the VAP cases captured by probable VAP. Such a substantial number of missed infections may lead to increased morbidity and mortality among children with TBI.(28, 34, 35)
VACO demonstrates superior specificity compared to VACMP, with values of 96% and 85%, respectively. The initial sacrifice in VACMP specificity, and therefore positive LR, does not persist throughout the algorithm. As intended, both the original and modified diagnoses of possible and probable VAP as defined by the VAE algorithm display excellent diagnostic capability.(30, 34–38) In this population of pediatric TBI patients with VAP prevalence of 33%, a diagnosis of VACO portends a post-test probability of 75%, doubling the chance of true disease.
The VAP and VAC positive cohorts were similar in all characteristics, consistent with previous publications and an important finding in light of the poorly sensitive adult VAC criteria.(28, 34, 39) The resemblance between VACO and VACMP cases suggests that our pediatric algorithm does not preferentially select for a unique cohort (i.e. younger or more severely injured), but simply increases sensitivity.
Review of false positive cases (VAC positive, VAP negative) highlights the propensity of the VAE framework to identify not only infectious, but other complications of mechanical ventilation.(37, 40) When combining VACO and VACMP, only 50% go on to meet criteria for an infectious condition (IVAC or possible VAP). Moreover, the radiographic effusions and contusions identified in approximately 25% of the VACs are arguably not attributable to ventilator mismanagement, but rather natural progression of underlying trauma. This finding echoes a prospective adult study which reported only 37% of VAC to be potentially preventable events.(36)
Outcomes, including mortality, did not differ significantly between children with VAP and VAC; both diagnoses were associated with longer duration of ventilation, hospital and ICU length of stay compared to those with no pulmonary condition. The 80% of VAP cases that did not exhibit sufficient respiratory compromise for a diagnosis of VACO still demonstrated poor outcomes, bringing to question the relevance of respiratory deterioration as a cornerstone in the new VAE scheme(38). Focus on ventilator changes may lead to a substantial number of missed cases and consequently, underestimation of morbidity attributable to infectious ventilator complications.
There has been some disagreement regarding outcomes of VAP versus VAE, which may be due to differing “gold standard” definitions of ventilator-associated pneumonia.(12, 20, 28, 34, 39) We utilized the more stringent current pediatric CDC PNU2 criteria, which not only utilizes subjective and objective findings of VAP, but also requires a positive microbiologic specimen. Of note, PBB has demonstrated equivalent sensitivity and improved specificity (up to 95%) when compared to BAL(41–43), thus should not be considered a limitation of this study.
There was a trend toward greater mortality among those without a pulmonary diagnosis when compared to those with VAC or VAP (any pulmonary diagnosis). This finding is likely attributable to survival bias, as those diagnosed with a pulmonary condition must live long enough to meet the respective criteria. Mortality difference between VACMP and VACO may be explained via similar principles. A patient does not have to live as long to meet VACMP, which only requires 1 day of sustained ventilator change (vs. 2 days for VACO). Lower rates of tracheostomy and more discharges to home (versus rehab/nursing care) in those patients not diagnosed with a pulmonary condition supports the hypothesis that the increased mortality is an unintended consequence of case definitions, rather than increased morbidity.
This single center observational study was limited by relatively small cohort size. The retrospective design is a strength, as it eliminates the possibility of manipulating ventilator settings to evade VAE diagnosis, a concern raised by other investigators.(28) Higher rates of VAP in our study cohort (TBI) prohibits extrapolation of the positive and negative predictive values to a conventional PICU population.(7, 11) Sensitivity and specificity are typically stable regardless of prevalence, and should therefore remain applicable. Lastly, ventilator management in brain-injured patients disallows permissive hypercarbia and lung-protective strategies in order to manipulate intracranial pressure, which may intrinsically alter sensitivity and specificity of the algorithm.(44)
Conclusion
We evaluated the new CDC/NHSN VAE criteria in a pediatric cohort and found that the adult VAE algorithm has poor sensitivity but good specificity compared to current PNU2 defined ventilator-associated pneumonia. Modified pediatric criteria improve sensitivity without a substantial decrease in specificity, but the algorithm is arguably still too insensitive to utilize for clinical surveillance. Implemented in current form, the VAE criteria would miss a large number of VAP diagnoses, which may result in higher pediatric ICU morbidity and mortality. The highly specific VAE VAP diagnoses may prove to be the most useful and objective quality measures for inter-institution comparison.
Acknowledgements
MC, MH, CS and SB had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. MC, MH, CS, TB and SB contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
Footnotes
Dr. Hamele is an active duty military officer. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the United States Government.
Author supported by NICHD K23 HD074620
Notation of prior abstract presentation:
AAP Experience, Section on Critical Care, October 12, 2014 in San Diego, California.
References
- 1.AHRQ Patient Safety Network - Health Care-Associated Infections. [[cited 2015 Mar 27]]; [Internet]. Available from: http://www.psnet.ahrq.gov/primer.aspx?primerID=7.
- 2.Prevent Health Care-Associated Infections (HAIs) Health.gov (ODPHP) [[cited 2015 Mar 2]]; [Internet]. Available from: http://www.health.gov/hai/prevent_hai.asp.
- 3.ASL. U. S. Efforts To Reduce Healthcare-Associated Infections. [[cited 2015 Mar 27]]; [Internet]. Available from: http://www.hhs.gov/asl/testify/2013/09/t20130924.html.
- 4.Foglia E, Meier MD, Elward A. Ventilator-associated pneumonia in neonatal and pediatric intensive care unit patients. Clin Microbiol Rev. 2007;20:409–425. doi: 10.1128/CMR.00041-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curley MA, Schwalenstocker E, Deshpande JK, et al. Tailoring the Institute for Health Care Improvement 100,000 Lives Campaign to pediatric settings: the example of ventilator-associated pneumonia. Pediatr Clin North Am. 2006;53:1231–1251. doi: 10.1016/j.pcl.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Bigham MT, Amato R, Bondurrant P, et al. Ventilator-associated pneumonia in the pediatric intensive care unit: characterizing the problem and implementing a sustainable solution. J Pediatr. 2009;154:582–587. e2. doi: 10.1016/j.jpeds.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 8.Safdar N, Dezfulian C, Collard HR, et al. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med. 2005;33:2184–2193. doi: 10.1097/01.ccm.0000181731.53912.d9. [DOI] [PubMed] [Google Scholar]
- 9.Kallel H, Chelly H, Bahloul M, et al. The effect of ventilator-associated pneumonia on the prognosis of head trauma patients. J Trauma. 2005;59:705–710. [PubMed] [Google Scholar]
- 10.Patrick SW, Tse A, Kleinman K, et al. Health care-associated infections among critically ill children in the US, 2007–2012. Pediatrics. 2014;134:705–712. doi: 10.1542/peds.2014-0613. [DOI] [PubMed] [Google Scholar]
- 11.Gupta S, Boville BM, Blanton R, et al. A multicentered prospective analysis of diagnosis, risk factors, and outcomes associated with pediatric ventilator-associated pneumonia. Pediatr Crit Care Med. 2015;16:e65–e73. doi: 10.1097/PCC.0000000000000338. [DOI] [PubMed] [Google Scholar]
- 12.CDC. Pneumonia (Ventilator-associated [VAP] and non-ventilator-associated Pneumonia [PNEU]) Event. [[cited 2015 Apr 26]];2015 [Internet]. Available from: http://www.cdc.gov/nhsn/PDFs/pscManual/6pscVAPcurrent.pdf.
- 13.Klompas M, Platt R. Ventilator-associated pneumonia--the wrong quality measure for benchmarking. Ann Intern Med. 2007;147:803–805. doi: 10.7326/0003-4819-147-11-200712040-00013. [DOI] [PubMed] [Google Scholar]
- 14.Klompas M. Advancing the science of ventilator-associated pneumonia surveillance. Crit Care. 2012;16:165. doi: 10.1186/cc11656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevens JP, Kachniarz B, Wright SB, et al. When policy gets it right: variability in U.S. hospitals’ diagnosis of ventilator-associated pneumonia. Crit Care Med. 2014;42:497–503. doi: 10.1097/CCM.0b013e3182a66903. [DOI] [PubMed] [Google Scholar]
- 16.Pugin J, Auckenthaler R, Mili N, et al. Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid. Am Rev Respir Dis. 1991;143:1121–1129. doi: 10.1164/ajrccm/143.5_Pt_1.1121. [DOI] [PubMed] [Google Scholar]
- 17.Fàbregas N, Ewig S, Torres A, et al. Clinical diagnosis of ventilator associated pneumonia revisited: comparative validation using immediate post-mortem lung biopsies. Thorax. 1999;54:867–873. doi: 10.1136/thx.54.10.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klompas M, Magill S, Robicsek A, et al. Objective surveillance definitions for ventilator-associated pneumonia. Crit Care Med. 2012;40:3154–3161. doi: 10.1097/CCM.0b013e318260c6d9. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi Y, Morisawa K, Klompas M, et al. Toward improved surveillance: The impact of ventilator-associated complications on length of stay and antibiotic use in patients in intensive care units. Clin Infect Dis. 2013;56:471–477. doi: 10.1093/cid/cis926. [DOI] [PubMed] [Google Scholar]
- 20.Klompas M, Khan Y, Kleinman K, et al. Multicenter evaluation of a novel surveillance paradigm for complications of mechanical ventilation. PLoS One. 2011;6:e18062. doi: 10.1371/journal.pone.0018062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magill SS, Klompas M, Balk R, et al. Executive summary: Developing a new, national approach to surveillance for ventilator-Associated events. Ann Am Thorac Soc. 2013;10:S220–S223. doi: 10.1513/AnnalsATS.201309-314OT. [DOI] [PubMed] [Google Scholar]
- 22.CDC. Ventilator-Associated Event (VAE) 2015:1–47. [Internet]. Available from: http://www.cdc.gov/nhsn/PDFs/pscManual/10-VAE_FINAL.pdf.
- 23.Wheeler DS, Wong HR, Zingarelli B. Pediatric Sepsis - Part I: “Children are not small adults!”. Open Inflamm J. 2011;4:4–15. doi: 10.2174/1875041901104010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klompas M. Complications of mechanical ventilation — The CDC’s new surveillance paradigm. N Engl J Med. 2013;368:1472–1475. doi: 10.1056/NEJMp1300633. [DOI] [PubMed] [Google Scholar]
- 25.Hutchison James S, MD, Ward Roxanne E, BA, Lacroix Jacques, MD, Hébert Paul C, MD, MHSc, Barnes Marcia A, PhD, Bohn Desmond J, MB, Dirks Peter B, MD, Doucette Steve, MSc, Fergusson Dean, PhD, Gottesman Ronald, MD, Joff Ari R, PD, Singh Ram N, MD, Skippen Peter W, MD for the HPHITI and the CCCTG. Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;358:2447–2456. doi: 10.1056/NEJMoa0706930. [DOI] [PubMed] [Google Scholar]
- 26.Adelson PD, Wisniewski SR, Beca J, et al. Comparison of hypothermia and normothermia after severe traumatic brain injury in children (Cool Kids): a phase 3, randomised controlled trial. Lancet Neurol. 2013;12:546–553. doi: 10.1016/S1474-4422(13)70077-2. [DOI] [PubMed] [Google Scholar]
- 27.Adelson PD, Ragheb J, Muizelaar JP, et al. Phase II clinical trial of moderate hypothermia after severe traumatic brain injury in children. Neurosurgery. 2005;56:740–754. doi: 10.1227/01.neu.0000156471.50726.26. [DOI] [PubMed] [Google Scholar]
- 28.Lilly CM, Landry KE, Sood RN, et al. Prevalence and test characteristics of National Health Safety Network ventilator-associated events. Crit Care Med. 2014;42:2019–2028. doi: 10.1097/CCM.0000000000000396. [DOI] [PubMed] [Google Scholar]
- 29.Alharfi IM, Stewart TC, Helali IA, et al. Infection rates, fevers, and associated factors in pediatric severe traumatic brain injury. J Neurotrauma. 2014;31:452–458. doi: 10.1089/neu.2013.2904. [DOI] [PubMed] [Google Scholar]
- 30.Skrupky LP, McConnell K, Dallas J, et al. A comparison of ventilator-associated pneumonia rates as identified according to the National Healthcare Safety Network and American College of Chest Physicians criteria*. Crit Care Med. 2012;40:281–284. doi: 10.1097/CCM.0b013e31822d7913. [DOI] [PubMed] [Google Scholar]
- 31.Taira BR, Fenton KE, Lee TK, et al. Ventilator-associated pneumonia in pediatric trauma patients. Pediatr Crit Care Med. 2009;10:491–494. doi: 10.1097/PCC.0b013e3181a3108d. [DOI] [PubMed] [Google Scholar]
- 32.SR R, JS R, AM G, et al. THe epidemiology of pediatric traumatic brain injury in minnesota. Arch Pediatr Adolesc Med. 2001;155:784–789. doi: 10.1001/archpedi.155.7.784. [DOI] [PubMed] [Google Scholar]
- 33.Hamele M, Stockmann C, Cirulis M, et al. Ventilator associated pneumonia in pediatric traumatic brain injury. J Neurotrauma. 2015 doi: 10.1089/neu.2015.4004. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoeppel CM, Eriksson E a, Hawkins K, et al. Applicability of the National Healthcare Safety Network’s surveillance definition of ventilator-associated events in the surgical intensive care unit. J Trauma Acute Care Surg. 2014;77:934–937. doi: 10.1097/TA.0000000000000425. [DOI] [PubMed] [Google Scholar]
- 35.Muscedere J, Sinuff T, Heyland DK, et al. The clinical impact and preventability of ventilator-associated conditions in critically ill patients who are mechanically ventilated. Chest. 2013;144:1453–1460. doi: 10.1378/chest.13-0853. [DOI] [PubMed] [Google Scholar]
- 36.Boyer AF, Schoenberg N, Babcock H, et al. A prospective evaluation of ventilator-associated conditions and infection-related ventilator-associated conditions. Chest. 2015;147:68–81. doi: 10.1378/chest.14-0544. [DOI] [PubMed] [Google Scholar]
- 37.Septimus E, Green L, Klompas M. Ventilator-associated events: a broader perspective. Crit Care Med. 2015;43:e59–e61. doi: 10.1097/CCM.0000000000000638. [DOI] [PubMed] [Google Scholar]
- 38.Klompas M. Ventilator-associated conditions versus ventilator-associated pneumonia: different by design. Curr Infect Dis Rep. 2014;16:430. doi: 10.1007/s11908-014-0430-0. [DOI] [PubMed] [Google Scholar]
- 39.Klouwenberg PMCK, Van Mourik MSM, Ong DSY, et al. Electronic implementation of a novel surveillance paradigm for ventilator-associated events feasibility and validation. Am J Respir Crit Care Med. 2014;189:947–955. doi: 10.1164/rccm.201307-1376OC. [DOI] [PubMed] [Google Scholar]
- 40.Magill SS, Rhodes B, Klompas M. Improving ventilator-associated event surveillance in the National Healthcare Safety Network and addressing knowledge gaps: update and review. Curr Opin Infect Dis. 2014;27:394–400. doi: 10.1097/QCO.0000000000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willson DF, Conaway M, Kelly R, et al. The lack of specificity of tracheal aspirates in the diagnosis of pulmonary infection in intubated children. Pediatr Crit Care Med. 2014;15:299–305. doi: 10.1097/PCC.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 42.Labenne M, Poyart C, Rambaud C, et al. Blind protected specimen brush and bronchoalveolar lavage in ventilated children. Crit Care Med. 1999;27:2537–2543. doi: 10.1097/00003246-199911000-00035. [DOI] [PubMed] [Google Scholar]
- 43.Gauvin F, Dassa C, Chaibou M, et al. Ventilator-associated pneumonia in intubated children: comparison of different diagnostic methods. Pediatr Crit Care Med. 2003;4:437–443. doi: 10.1097/01.PCC.0000090290.53959.F4. [DOI] [PubMed] [Google Scholar]
- 44.Kochanek PM, Carney N, Adelson PD, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents--second edition. Pediatr Crit Care Med. 2012;13:S1–S82. doi: 10.1097/PCC.0b013e31823f435c. [DOI] [PubMed] [Google Scholar]