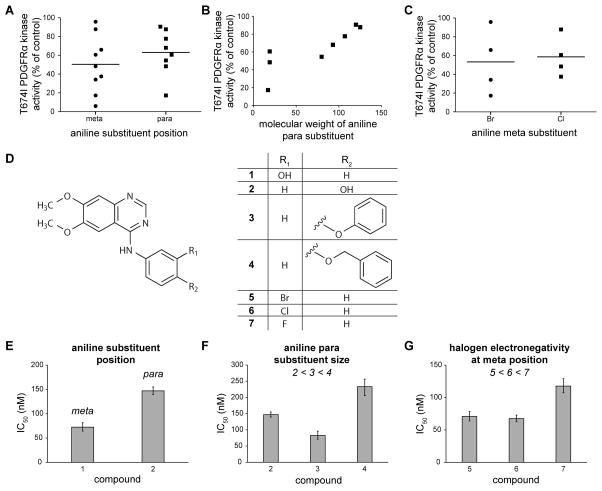
Figure 6. Chemical modifications affecting potency of 4-anilinoquinazoline compounds toward T674I PDGFRα.
(A) Remaining kinase activity of T674I PDGFRα in the presence of 4-anilinoquinazolines with substituents in either the meta or para position of the aniline ring. Horizontal lines represent the mean remaining kinase activity for the grouped data.
(B) Remaining kinase activity of T674I PDGFRα in the presence of 4-anilinoquinazolines containing a para substituent plotted against the molecular weight of the substituent.
(C) Remaining kinase activity of T674I PDGFRα in the presence of 4-anilinoquinazolines with halogen substituents in either the meta or para positions of the aniline ring. Horizontal lines represent the mean remaining kinase activity for the grouped data.
(D) Test compounds used to validate predictions of the 4-anilinoquinazoline pharmacophore hypothesis.
(E)–(G) IC50 values measured from dose response curves of compounds in (D) against T674I PDGFRα in vitro, grouped according to prediction tested: (E) substituents at the meta position of the aniline ring are favored over the same substituents at the para position, (F) large substituents at the para position of the aniline ring decrease potency, and (G) smaller/more electronegative halogen substituents at the meta position of the aniline ring decrease potency toward T674I PDGFRα. Dose response measurements were performed in triplicate. Error bars represent 95% confidence intervals. IC50 values are given in Table S3.