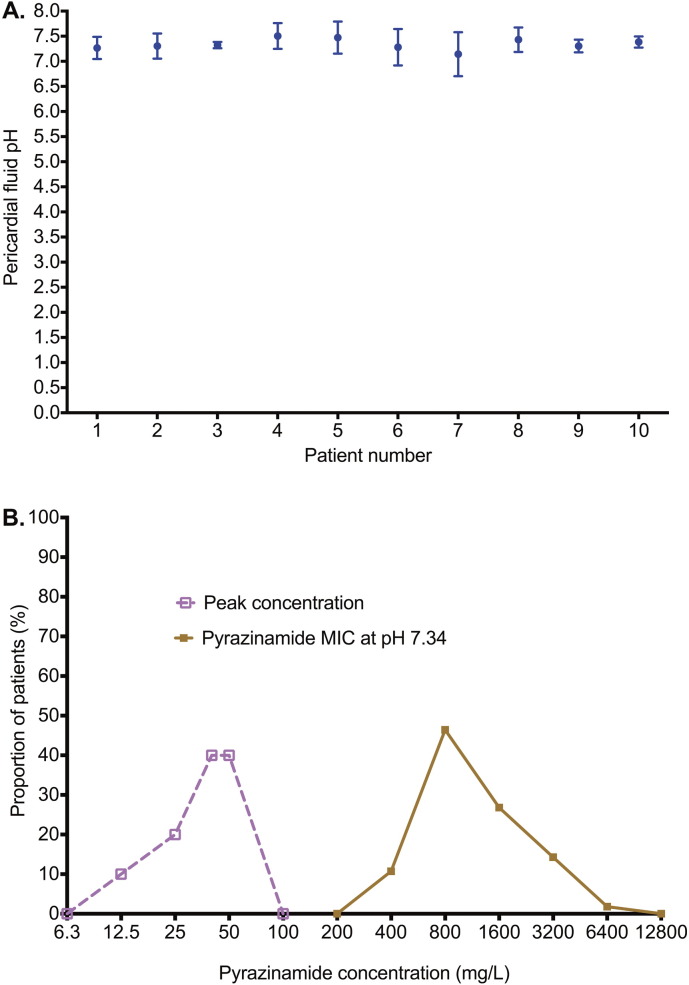
Fig. 6.
The pH of pericardial fluid and pyrazinamide MICs.
A. The pH in pericardial fluid in each patient was measured 8 times in triplicate; shown are the mean values and standard deviation. The D'Agostino and Pearson omnibus normality test p-value was 0.991, which means that the pH values in the pericardial fluid were normally distributed. All the pH values were greater than 7.0, and indeed were similar to the pH of 7.365 in the blood, which is slightly alkaline.
B. Pyrazinamide MICs measured at pH 5.9 were used calculate the actual MICs at physiological pH based on the relationship between the active moiety pyrazinoic acid (POA; anion POA−) and HPOA in the Handersen–Hasselbach equation (POA pKa = 2.9), as demonstrated by Zhang et al. (2002). At pH 5.90 the % of the HPOA is 0.1% compared to 0.00363% at pH 7.34, a 27.54-fold change, which is the factor used to calculate the MIC value at pH 7.34 based on that identified at pH 5.9. This leads to an MIC with a median 18.6-fold higher than the median peak concentrations; indeed the highest pyrazinamide peak concentration was 4-fold lower than the lowest MIC.