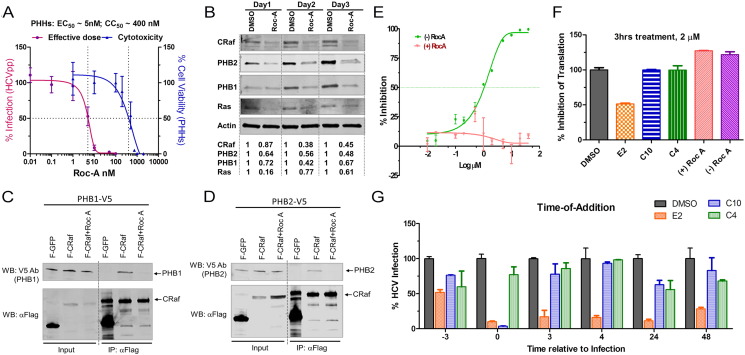
Fig. 3.
Roc-A potently inhibits HCV infection.
(A) PHHs seeded in a 48-well plate were treated with Roc-A or DMSO and then infected by HCVpp. EC50 and CC50 (50% cytotoxicity dose) were calculated based on the fitted Sigmoid curves.
(B) Huh7.5.1 cells were treated with Roc-A (20 nM) or DMSO for 1, 2, and 3 days. Cellular PHB1, PHB2, CRaf, and Ras were detected by Western blotting. Numbers shown below Western blot gel images indicate the relative protein levels quantified by Odyssey imaging system (LI-COR Biosciences).
(C–D) Flag-CRaf (F-CRaf) and V5-tagged PHB1 & 2 were co-transfected into 293T cells and then left untreated or treated with Roc-A (20 nM) before immunoprecipitation with anti-Flag antibody. The pulled-down PHB1 & 2 as well as CRaf were detected by immunoblotting.
(E) Different inhibitory profiles of the (+)- and (−)-Roc-A enantiomers on HCVcc infection.
(F) A CMV-luciferase reporter construct was transfected into Huh7.5.1 cells. 24 h post-transfection each compound was added to the cells for 3 h at 2 μM. After removal of the compound, the cells were further incubated for 24 h followed by luciferase assay.
(G) C10 (racemic aglaroxin C) inhibited HCV infection when added together with the virus. HCVcc-Luc was added to Huh7.5.1 cells at 37 °C and incubated for 3 h. At the indicated time points, 2 μM of each compound or DMSO was added into the media and incubated for 3 h prior to removal. Infected cells were incubated at 37 °C for an additional 48 h prior to luciferase assay (mean of n = 3; error bars, s.d.). Compound E2 (−)-NH hydroxamate, is a potent translation inhibitor (Rodrigo et al., 2012) and hence appeared to inhibit HCV no matter when added; compound C4 (±)-β-lactone (Lajkiewicz et al., 2014), was added as a negative control as it exerted negligible effect.