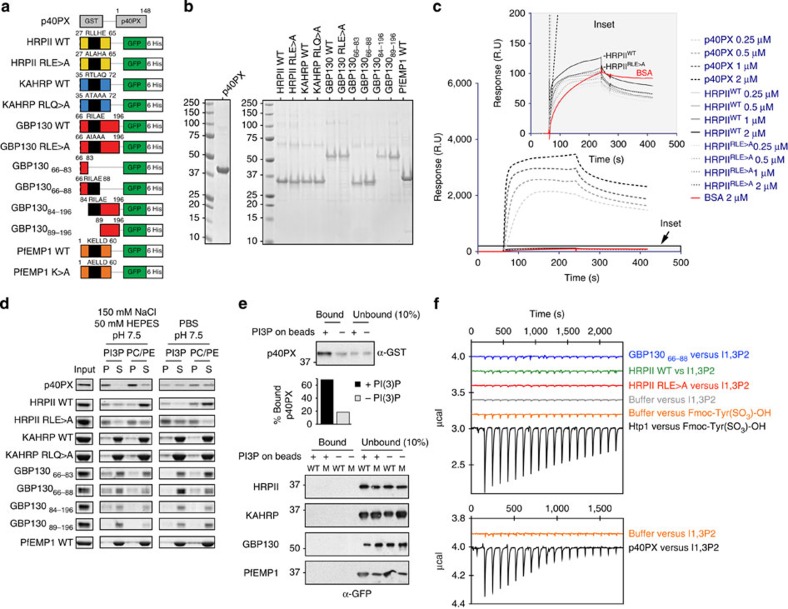
Figure 1. Exported P. falciparum proteins do not bind PI(3)P.
(a) Recombinant proteins expressed in this study as fusions to GST or GFP/6His. (b) Recombinant proteins stained with Coomassie blue stain. Molecular weights (kDa) are shown. (c) Binding of p40PX and HRPII with native PEXEL (WT) or mutant PEXEL (RLE>A) to PI(3)P liposomes measured by SPR. Binding to control PC/PE liposomes was subtracted to give the sensorgrams24. BSA was used to measure nonspecific binding to the liposome-coated chip (red). Inset: a similar level of low binding of HRPIIWT and HRPIIRLE>A to PI(3)P liposomes. Experiments were performed in triplicate. (d) Binding of proteins to PI(3)P liposomes or control PC/PE liposomes, determined by ultracentrifugation. Pellet and supernatant fractions were resolved by SDS–PAGE and Coomassie blue stain. Proteins were ultracentrifuged in buffer to remove potential aggregates before incubation with liposomes39, explaining why input and sum of pellet and supernatant sometimes differ. Experiments were performed in triplicate. Full-length gels shown in Supplementary Fig. 6. (e) Binding of p40PX and P. falciparum proteins with native PEXEL (WT) or mutant PEXEL (M) to PI(3)P-coated beads (+) or control beads lacking lipid (−). Bound protein was eluted in SDS–PAGE sample buffer and detected by immunoblot with anti-GST (p40PX) or anti-GFP (P. falciparum proteins). Ten per cent of unbound fraction volume was loaded to visualize protein inputs. Densitometry (histogram shown) of the p40PX bands shows that 68% of p40PX input bound to PI(3)P-coated beads (PI3P+) compared with 18% of input to control beads (PI3P−). No binding was detected for P. falciparum exported proteins. Experiments were performed in triplicate. Full-length gels shown in Supplementary Fig. 7. (f) Binding of recombinant proteins to phospholipid head group of PI(3)P (inositol 1,3 bisphosphate; I1,3P2) in solution, measured by isothermal calorimetry. The titration did not differ between I1,3P2 (2.4 mM) into dialysis buffer (150 mM NaCl, 20 mM HEPES pH 7.4) (negative control) and the same buffer containing either GBP13066–88, HRPIIWT or HRPIIRLE>A. As controls, binding of Htp1 from S. parasitica to Fmoc-Tyr(SO3)-OH and also p40PX binding to I1,3P2 are shown. Experiments were performed in duplicate.