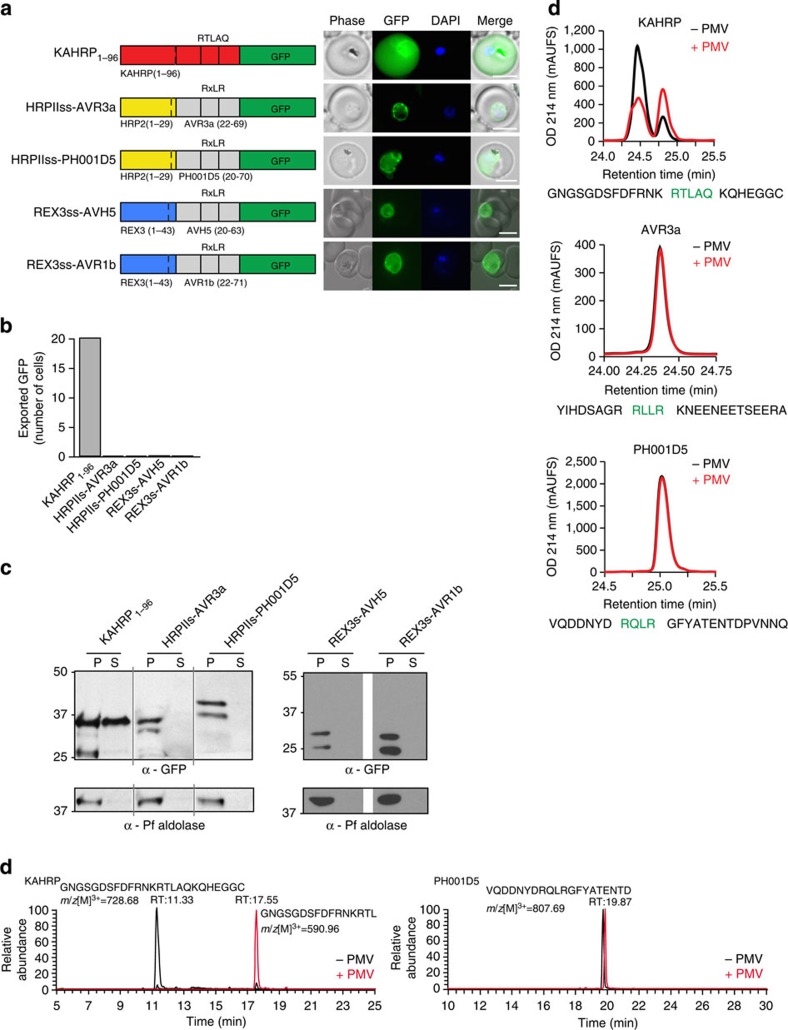
Figure 3. Oomycete RxLR does not mediate export in P. falciparum.
(a) KAHRP1–96 is exported to the P. falciparum-infected erythrocyte; however, HRPIIss-AVR3a, HRPIIss-PH001D5, REX3ss-AVH5 and REX3ss-AVR1b are not exported but accumulate in the parasite and PV. Scale bar, 5 μm. (b) Quantification of cells with exported GFP determined using 20 P. falciparum-infected erythrocytes per construct (10 cells per experiment, performed twice). (c) Immunoblot with anti-GFP antibodies of each chimera from the tetanolysin pellet (P) and supernatant (S) shows that KAHRP1–96 is exported but RxLR chimeras are not. Tetanolysin inserts pores in the erythrocyte membrane, allowing sampling of the host cell cytosol. Aldolase was included as a control, as described previously71. Full-length gels shown in Supplementary Fig. 8. (d) RP-HPLC shows the KAHRP peptide is cleaved when incubated with PMV (red). AVR3a and PH001D5 peptides are not cleaved by PMV. PEXEL/RxLR sequences are labelled green. (e) MS/MS-extracted ion chromatograms of KAHRP and PH001D5 peptides after incubation with PMV shows that KAHRP peptides were cleaved after the PEXEL Leu (GNGSGDSFDFRNKRTL−) but PH001D5 was not cleaved.