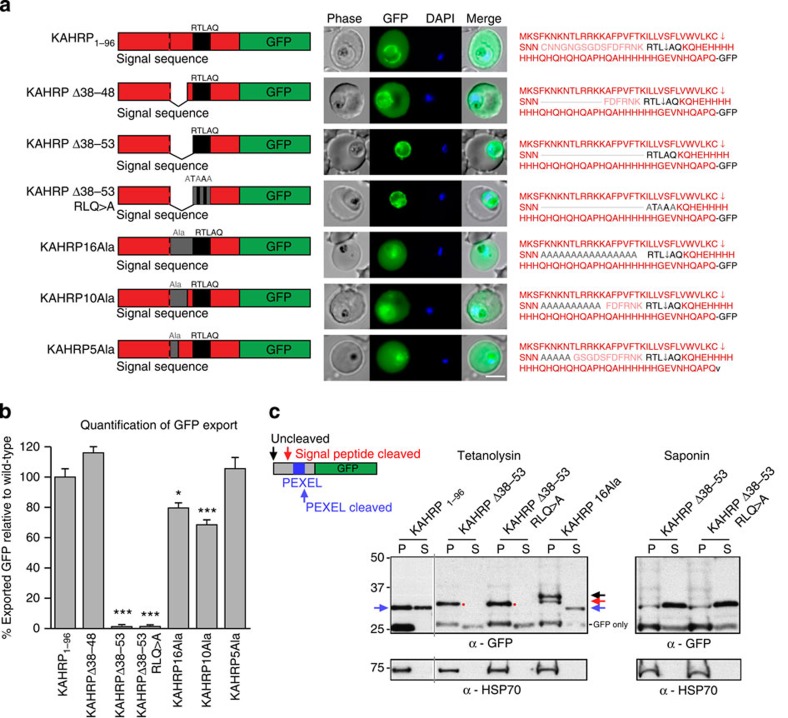
Figure 5. Correct PEXEL positioning is essential for export.
(a) KAHRP1–96 and KAHRPΔ38–48 are exported to the P. falciparum-infected erythrocyte but KAHRPΔ38–53 and KAHRPΔ38–53 RLQ>A accumulate in the PV and are not exported. KAHRP16Ala, KAHRP10Ala and KAHRP5Ala are exported to the red blood cell. Scale bar, 5 μm. (b) Quantification of export in a. Data are the mean (±s.e.m.) GFP fluorescence intensity in the host cell from 20 cells per construct (20 replicates), shown as a percentage of KAHRP1–96. Data were analysed by t-test (***P<0.0001 and *P<0.003, compared with KAHRP1–96). (c) Immunoblot with anti-GFP antibodies of chimeras from the tetanolysin and saponin pellet (P) and supernatant (S). This shows KAHRP1–96 at a size corresponding to cleavage at the PEXEL and it was exported (32 kDa; blue arrows), KAHRPΔ38–53 and KAHRPΔ38–53 RLQ>E were cleaved to a size corresponding to cleavage by signal peptidase (32.5 kDa; red spots) and were not exported, but were present in the saponin supernatant, confirming they were secreted to the PV, and that KAHRP16Ala was of a size corresponding to cleavage at the PEXEL and it was exported (32 kDa; blue arrow); however, cleavage was inefficient as bands consistent with signal sequence cleaved (red arrow) and uncleaved (black) species were present in the pellet fraction. HSP70 was used as a control. Full-length gels shown in Supplementary Fig. 10.