Figure 1. Physical interaction between sMEK1 and VEGFR-2.
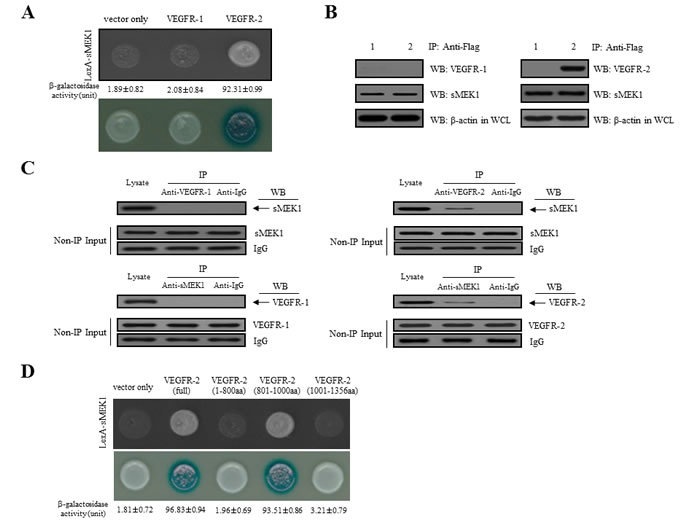
A. Positive interactions were confirmed by observed cell growth on a medium lacking leucine and by the formation of blue colonies on X-gal plates containing 2% galactose. β-galactosidase activity (unit), measured by adding o-nitrophenyl β-D-galactopyranoside (ONPG), is shown below the corresponding lanes. B. The co-immunoprecipitation of sMEK1 with VEGFR-1 or VEGFR-2. Immunoprecipitation from transfected HEK293T cells was performed using anti-FLAG antibodies in lysates, followed by immunoblotting with anti-sMEK1, anti-VEGFR-1, and anti-VEGFR-2 antibodies. C. Endogenous proteins in total lysates of HEK293T cells were subjected to co-immunoprecipitation (IP) with an antibody as indicated followed by Western blotting (WB) with an anti-sMEK1 or anti-VEGFR-2 antibody. A rabbit IgG and VEGFR-1 were included as an IP negative control. The input (Non-IP) WB data indicated the integrity of lysates used for IP. D. Mapping of the VEGFR-2 region critical for the interaction with sMEK1 using a protein-protein interaction assay in vivo. The cDNA constructs were co-transformed into EGY48 yeast cells, and protein-protein interactions were assessed using a yeast two-hybrid system. Positive interactions were confirmed by observed cell growth (upper panel) and the formation of blue colonies (lower panel). The β-galactosidase activity of each construct in negative controls (vector only) was < 1.81±0.72. Data are representative of three independent experiments and are presented as means±SDs.
