Figure 3. The susceptibility of PrAg-PCIS to proteolytic cleavage by hepsin and matriptase is consistent with their abilities to cleave the RCL of PCI to form protease-serpin inhibitory complexes.
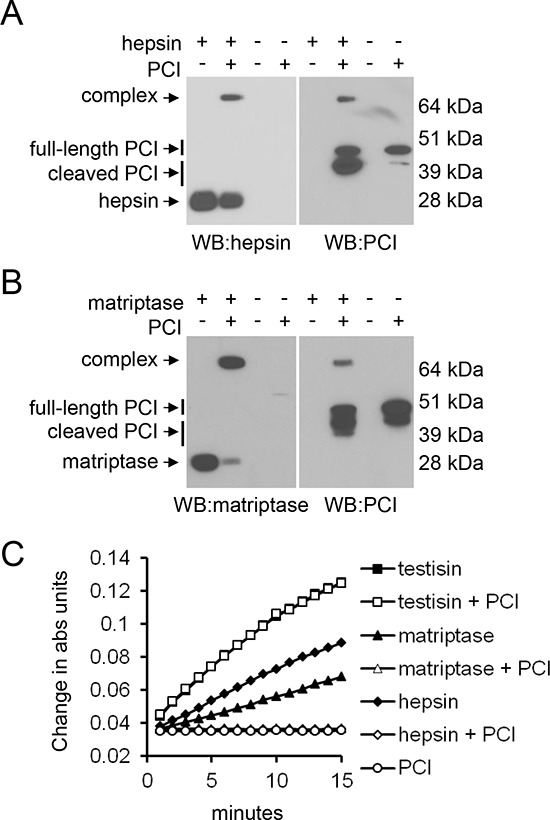
A. Recombinant hepsin or B. recombinant matriptase were incubated with PCI, at room temperature prior to immunoblotting with anti-PCI, anti-hepsin, or anti-matriptase antibodies. Full-length PCI, cleaved PCI, and serpin-protease inhibitory complexes are as indicated. Each blot is representative of at least two independent experiments. C. PCI inhibits hepsin and matriptase catalytic activities. Recombinant testisin, hepsin, and matriptase were incubated with the peptide substrate, Suc-AAPR-pNA, in the presence or absence of PCI and the changes in absorbance monitored over the course of 15 minutes. The data is representative of at least two independent experiments.
