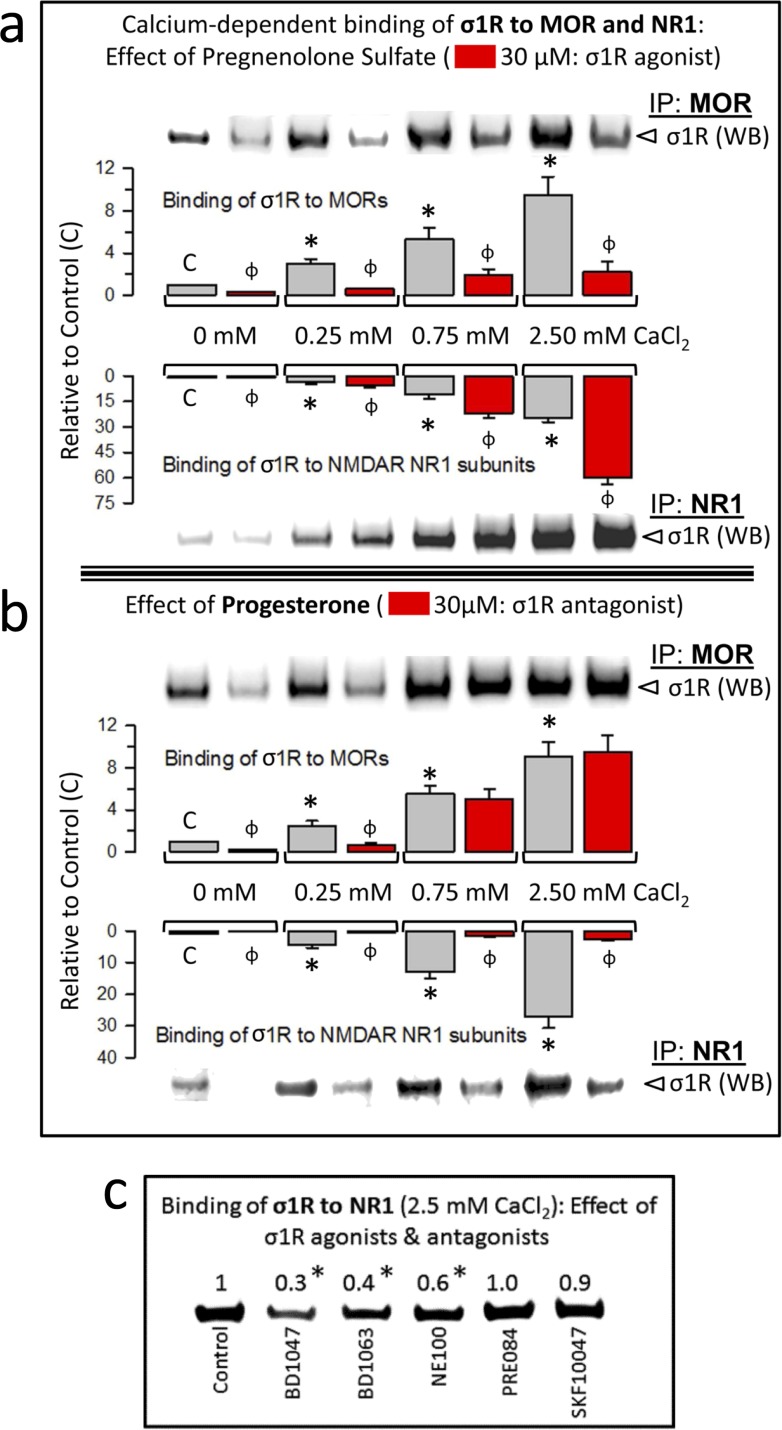
Figure 6. Calcium-dependent binding of σ1Rs to MORs and NR1 subunits: Influence of σ1R regulation.
a. The σ1R agonist pregnenolone sulfate stabilizes the σ1R-NR1 interaction while diminishing σ1R binding to MORs. The recombinant MOR, NR1 C0-C1-C2 and σ1R were used at 100 nM. The assay was performed in the presence of increasing amounts of calcium chloride (0, 0.25, 0.75, 2.5 mM). Bait proteins (GST-NR1 C0-C1-C2 and GST-MOR) were immobilized by covalent attachment to NHS-activated Sepharose. Prey proteins alone did not bind either to the NHS-Sepharose or to the recombinant GST (negative controls). The pellets obtained were processed as described to determine σ1Rs in Western blots (see the Methods section). The bars are the mean ± S.E.M of three independent assays. Effect of calcium. For each interaction of σ1R, MOR-σ1R and NR1-σ1R, the effects of increasing calcium availability are shown relative to the data obtained in the absence of calcium control group (C): arbitrary value of 1): *Significant differences, ANOVA (DF = 11), Dunnett multiple comparisons vs control group, p < 0.05. Effects of σ1R agonism (bars in red). These effects are indicated for each interaction of σ1R and calcium concentration studied: ɸ significant difference between the paired groups at each calcium concentration studied, with and without the sigma ligand; ANOVA (DF = 23) all pairwise Holm-Sidak multiple comparison test, p < 0.05. b. While diminishing σ1R binding to NR1 subunits, Progesterone, a σ1R antagonist, has a calcium-dependent effect at σ1R-MOR complexes. Details as in (a). c. Effect of exogenous (synthetic) ligands of σ1Rs on the NR1-σ1R complex: agonists, PRE084 and SKF10047; antagonists, BD1047, BD1063 and NE100. *Significantly different from the control group that received saline instead of the σ1R ligand; ANOVA (total DF = 11), Dunnett multiple comparisons vs control group, p < 0.05.