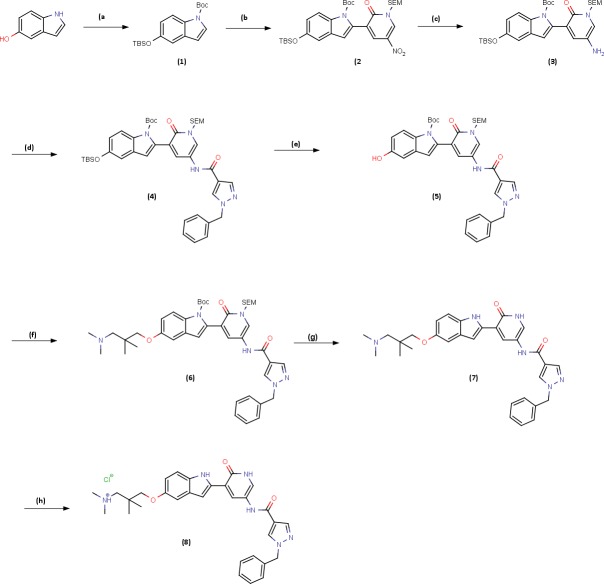
Figure 7. Scheme 1: Synthesis route of V158411.
Reagents and conditions: A. TBDMSCl, DIPEA, DMAP, DCM, rt, 3 h then (BOC)2O, DMAP, DCM rt 2 h, 100%; B. LDA, (iPrO)3B, THF, 0-5°C, 30 min then Intermediate I., K2CO3, Pd(dppf)Cl2:CH2Cl2, 60°C, 2 h, 76%; C. 10%Pd/C, HCO2NH4, MeOH 60°C, 1h, 100%; D. Intermediate (ii), Et3N, DCM, 0°C then rt, 18 h, 94%; E. TBAF, 1.0M in THF, THF, 0°C then rt, 2 h, 93%; F. Intermediate (iii), Cs2CO3, DMF, 100°C, 4 h, 84%, G. TBAF, 1.0M in THF, ethylenediamine, THF, 70°C, 18 h, 81%; H. HCl solution, 2.0M in Et2O, MeOH/CHCl3, 0°C then rt, 1h, 69%.