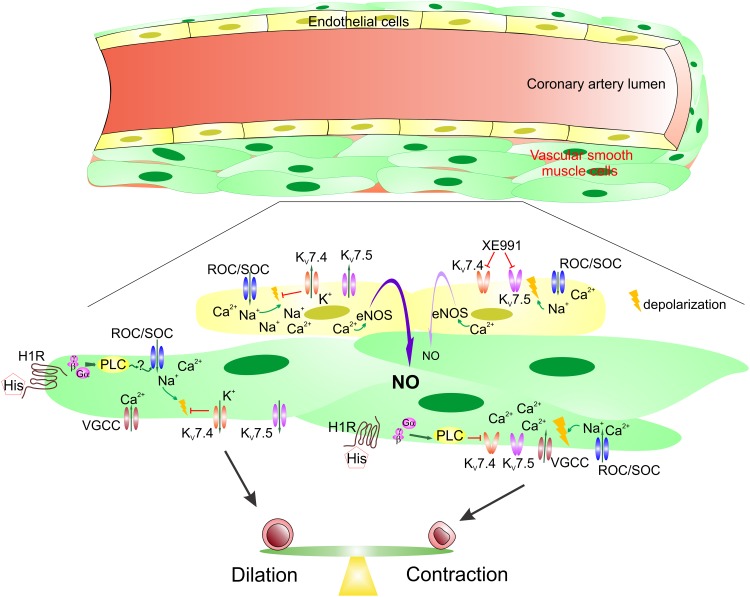
Fig 6. Diagram summarizing the roles of Kv7 channels in the pig right coronary artery.
It appears that a balance between Kv7 channel activation and inhibition contributes to sculpturing the shape of histamine-induced CA contractile responses. Histamine acts on the histamine H1 receptor (H1R) expressed in CA smooth muscle cells. This results in a phospholipase C (PLC)-dependent stimulation of receptor-operated and store-operated channels (ROC/SOC) mediating cation influx that depolarizes the smooth muscle cells. In turn, smooth muscle cell depolarization activates voltage-gated Ca2+ channels that leads to massive Ca2+ influx into the smooth muscle cells with a consecutive smooth muscle contraction and further depolarization. Such stronger depolarization stimulates Kv7 activity repolarizing smooth muscles and weakening coronary artery ring contractions. Conversely, histamine-dependent inhibition of Kv7 channels via a PLC-dependent mechanism leads to an increased smooth muscle cell depolarization and strengthens ring contractions. In endothelium, bradykinin-dependent ROC/SOC activation also results in Na+ and Ca2+ influx that is driven by the gradient of these cations and negative potential inside endothelial cells. However, such cation influx depolarizes endothelial cells reducing the driving force. Activation of endothelial Kv7 channels likely enhances endothelium-dependent dilatory responses in the pig right coronary artery by maintaining more negative potential inside endothelial cells that results in greater Ca2+ influx known to stimulate Ca2+-dependent nitric oxide synthase (eNOS) causing increased NO production.