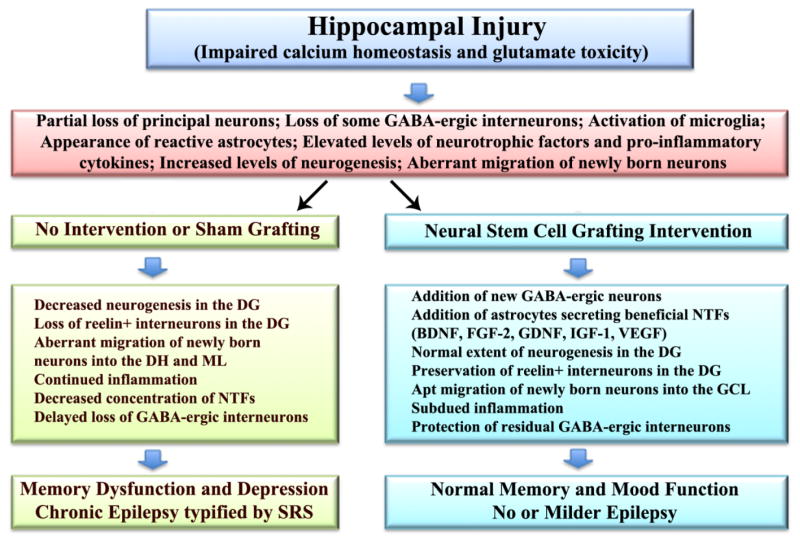
Figure 4.
Potential mechanisms by which neural stem cell grafting intervention into the hippocampus in the early phase after injury modulates hippocampal post-injury plasticity in the intermediate and chronic phases after injury, promotes the maintenance of normal memory and mood function, and restrains the development of chronic epilepsy. An initial injury to hippocampus following head trauma, ischemia, stroke or status epilepticus likely involves impaired neuronal calcium homeostasis and glutamate toxicity. In the acute phase after injury, this leads to partial loss of principal neurons and gamma-amino butyric acid (GABA) positive interneurons, activation of microglia, appearance of reactive astrocytes, increased concentrations of neurotrophic factors and pro-inflammatory cytokines, and enhanced but abnormal neurogenesis typified by aberrant migration of newly born neurons. Absence of therapeutic intervention in the acute phase (left half of the figure) leads to multiple adverse changes in the intermediate and chronic phases of injury, which comprise decreased neurogenesis, loss of reelin+ interneurons in the dentate gyrus (DG), abnormal migration of newly born neurons into the dentate hilus (DH) and the molecular layer (ML), sustained inflammation, decreased concentration of multiple neurotrophic factors (NTFs) and further loss of GABA positive interneurons. All of these changes are known to contribute towards cognitive and mood dysfunction and chronic epilepsy development seen in the chronic phase after injury. However, neural stem cell grafting intervention in the acute phase after injury (right half of figure) considerably modulates these adverse effects with the addition of new GABA-ergic neurons and astrocytes secreting a variety of neurotrophic factors, which in turn contribute to the maintenance of normal memory and mood function and no or milder epilepsy typified by diminished frequency and intensity of spontaneous recurrent seizures (SRS). BDNF, brain derived neurotrophic factor; FGF-2, fibroblast growth factor-2; IGF-1, insulin-like growth factor-1; VEGF, vascular endothelial growth factor.