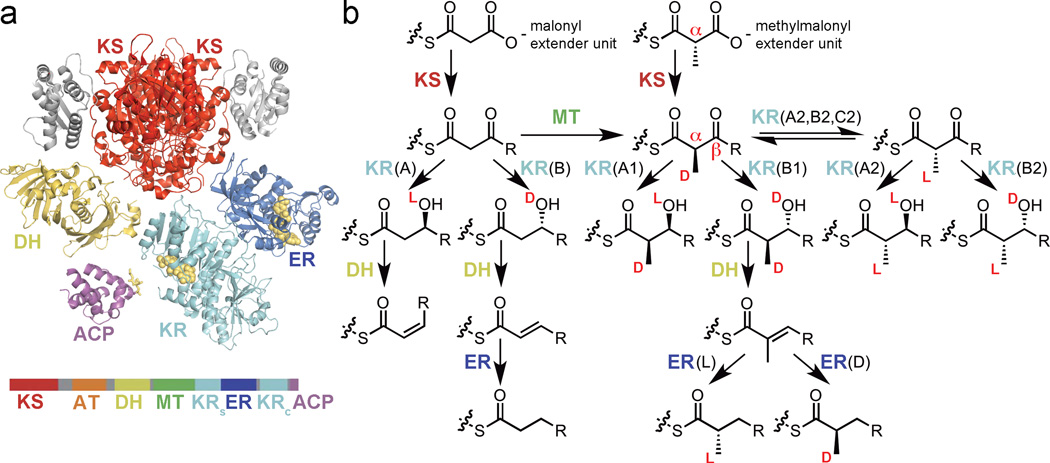
Figure 1.
Modular PKS enzymes and the stereocenters they set. (a) Crystal structures of a representative KS, KR, DH, and ER domain (PDB: 4NA2, 3SLK, 3EL6) as well as an NMR structure of a representative holo-ACP (PDB: 2LIW) are shown above the primary structure of a full cis-AT PKS module (~2500 aa). Note the 18-Å phosphopantetheinyl arm of ACP. The methyltransferase (MT) domain is largely uncharacterized and will not be discussed here. (b) Extender units, selected by ATs, are condensed with polyketide intermediates by KSs. The elongation reaction and subsequent processing reactions are exquisitely stereocontrolled (stereochemical orientations are indicated). Any one of the displayed ketide units can by added by a PKS module containing the appropriate enzymes. All molecules are bound to an ACP. Enzyme types are defined in the text.