Figure 4.
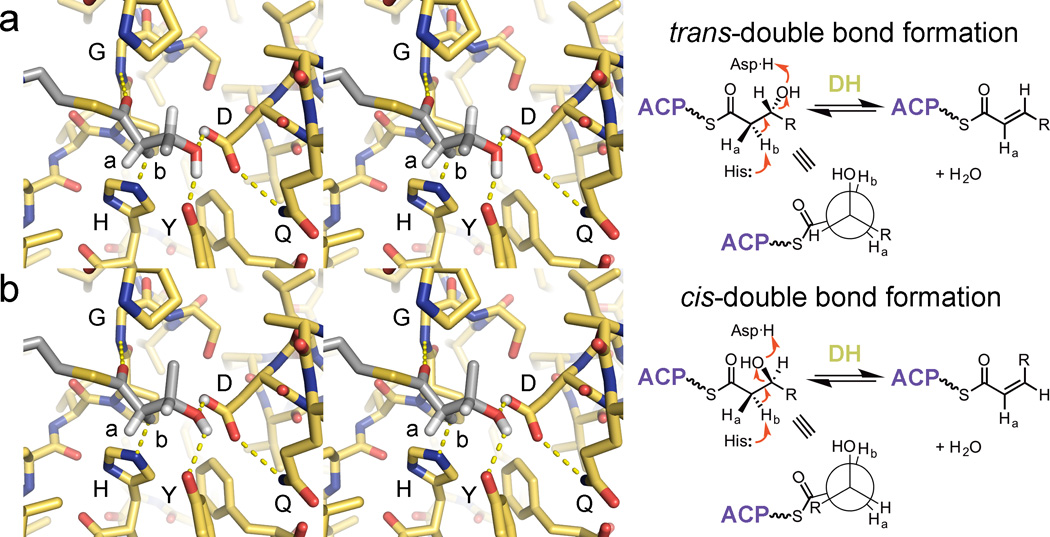
DH double bond formation. (a) Dehydration of a substrate to yield a trans-double bond is thought to occur through the syn-coplanar elimination of its d-β-hydroxyl group and l-α-proton (Hb). For this to occur, DH must bind its substrate so that its α- and β-substituents eclipse one another. All reported DH structures show the same orientation of the catalytic histidine and aspartic acid as well as the glycine NH; the tyrosine is also highly conserved (PDB: 3EL6). (b) The syn-coplanar dehydration reaction to generate a cis-double bonded product from an l-β-hydroxyacyl substrate is most likely catalytically equivalent to the syn-coplanar dehydration reaction that generates a trans-double bonded product from a d-β-hydroxyacyl substrate. DHs that generate cis-double bonds must accommodate for the different chain geometry.
