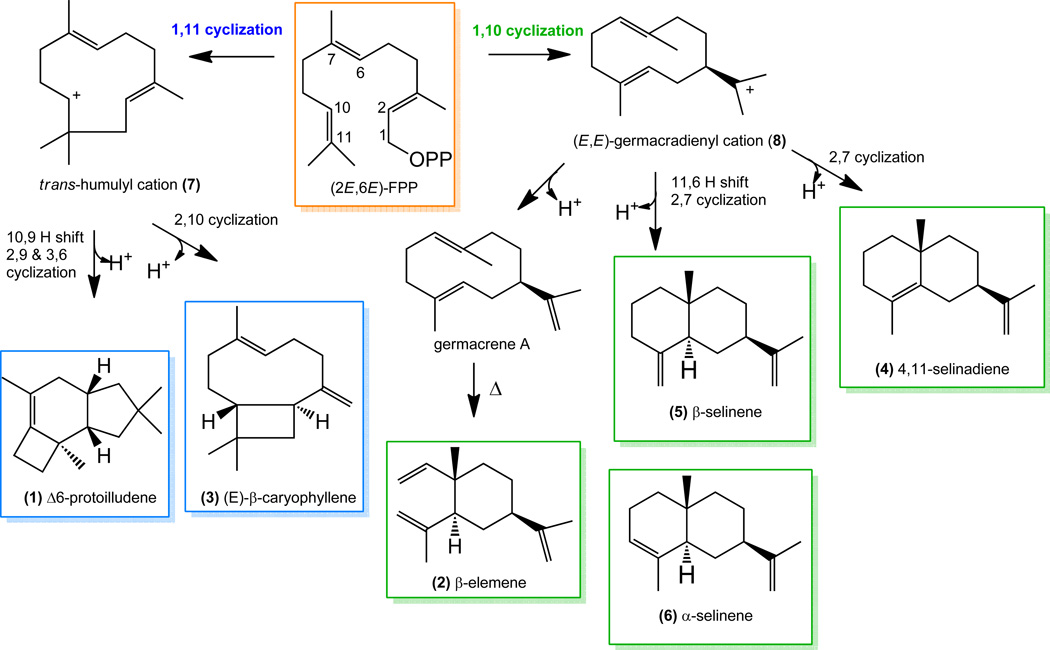
Scheme 1. Proposed mechanisms for sesquiterpene production by Δ6-protoilludene synthases.
Metal-dependent ionization results in the release of the diphosphate moiety (OPP) from farnesyl pyrophosphate ((2E,6E)-FPP) leading to either a 1,11 or 1,10 cyclization of the primary carbocation. A 1,11 cyclization leads to the trans-humulyl cation (7); a 1,10 cyclization of the primary carbocation leads to the (E,E)-germacradienyl cation (8). Further hydride shifts and cyclizations results in final sesquiterpene products (1-6).