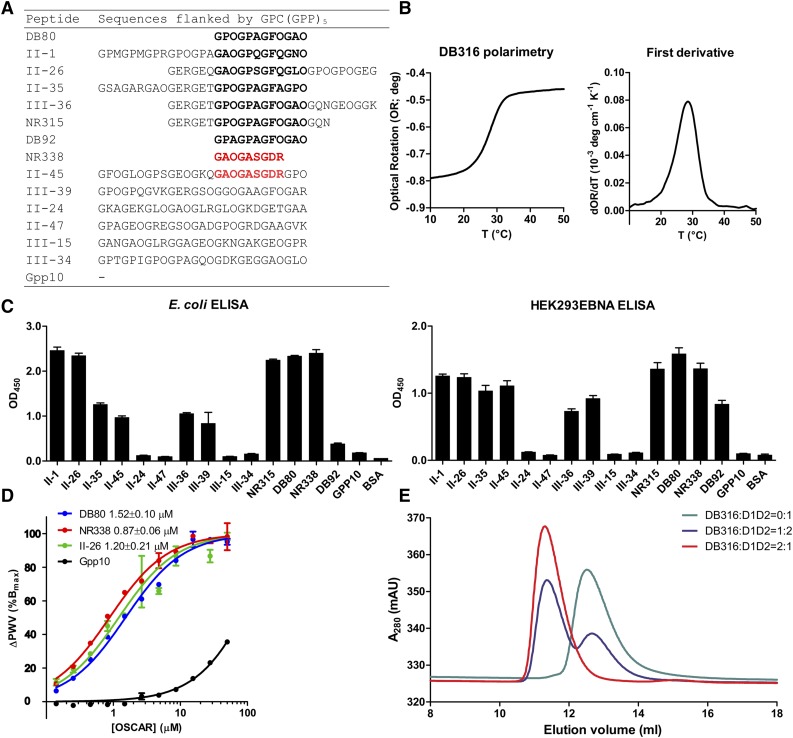
Figure 1.
Design of a consensus collagen triple-helical peptide. (A) Table of collagenous peptide sequences used in solid-phase binding experiments. Collagen Toolkit peptides are identified by their numbers from Toolkits II and III, whereas derivative peptides are identified by their laboratory reference numbers. The consensus motifs, GXOGPXGFX or GAOGASGDR, are shown in black or red font, and segments of sequence that surround them are shown in gray. Not shown are the N- and C-terminal flanking motifs, GPC[GPP]5- and -[GPP]5GPC-amide, which are present on each peptide. Gpp10 denotes the control, GPC[GPP]10GPC-amide. (B) Polarimetry data of peptide DB316, Acetyl-GPOGPOGPOGPAGFOGPOGPO-amide were collected from 10°C to 70°C (up to 50°C is shown) at a ramp rate of 1°C min−1 as described in the supplemental Methods. First derivative of the original optical rotation data were used to define the peptide Tm. (C) Solid-phase binding of recombinant OSCAR (ectodomain E coli– or HEK293EBNA-expressed, 0.1 µg per well) on the collagenous peptides listed in panel A. Each peptide/protein combination was tested in triplicate, as described in supplemental Methods, and the inert peptide, (GPP)10, and BSA were used as negative controls. Data shown are mean ± SEM. (D) Biosensor curves of increasing concentrations of E coli–expressed recombinant OSCAR ectodomain titrated onto SRU 96-well plate coated by DB80, NR338, II-26, as well as (GPP)10 as negative control. Each point was collected in triplicate, and each data set was fitted to a model of specific and nonspecific binding in GraphPad Prism. The nonspecific binding curves were then removed for clarity. (E) Analytical gel filtration of recombinant OSCAR ectodomain preincubated with DB316 in different molar ratios. Each curve was derived separately using a Superdex 75 10/300gl column, running at 0.2 mL per minute, as described in supplemental Methods, and superimposed into 1 figure. Bmax, maximum binding; deg, degrees; dOR/dT, rate of change of optical rotation with temperature; ELISA, enzyme-linked immunosorbent assay; mAU, milli absorbance units; OD, optical density; PWV, peak wavelength; SEM, standard error of the mean.